Improving VOC biomarker discovery for gut disorders
Accommodating gut microbial influences on a properly designed study is key
| Publication information: K. Malderen et al. Volatomics in inflammatory bowel disease and irritable bowel syndrome, EBioMedicine. (2020) 54:102725. doi: 10.1016/j.ebiom.2020.102725
Disease Area: Irritable bowel syndrome, Inflammatory bowel disease, Gut microbiome, Gastrointestinal Application: Disease diagnosis, Disease monitoring, Differential diagnosis, Personalized medicine Sample medium: Breath, Fecal, Urine
Summary:
|
Despite presenting similar symptoms with chronic abdominal pain and stool pattern changes, irritable bowel syndrome (IBS) and inflammatory bowel disease (IBD) have different pathogenic mechanisms – the former most likely caused by multiple factors, including physiological and psychological influences, and the later by chronically relapsing inflammation. To date, differential diagnosis between IBS and IBD or disease subtypes still require invasive biopsies, which are expensive and painful. A non-invasive approach, with the potential of lower cost, is therefore an appeal for disease diagnostic and monitoring.
In recent years, mechanistic studies of the pathogenesis of IBS and IBD have identified multiple biological pathways contributing to these conditions, including inflammatory signaling and activity of the gut microbiome. Microbial metabolism, as well as physiological processes occurring in the human body can both produce volatile organic compounds (VOCs). VOCs can be collected non-invasively and analyzed from multiple sample types, including breath or the headspace of urine or fecal samples. Analysis of these VOCs, termed volatomics, has gained interest for its potential of developing non-invasive biomarkers in multiple clinical settings, including diagnosis of IBS and IBD.
Malderen et al. reviewed and analyzed 25 volatomic studies published between 1993-2018, eight of them with IBS subjects and 17 with IBD subjects. Their review summarized the current knowledge of using VOCs (breath, feces, urine) for diagnosis of IBS and IBD, for differentiation between IBS and IBD or disease subtypes, disease states, monitoring treatment responses and the relation between gut microbiome and VOC composition. The VOCs differentiated between comparison groups in these studies varied widely, which could be due to several reasons including lack of standardization in sample collection and different analytical methods used. Therefore, only compounds described in more than one study were being discussed further in the review.
Promising VOC biomarkers and the importance of gut microbes
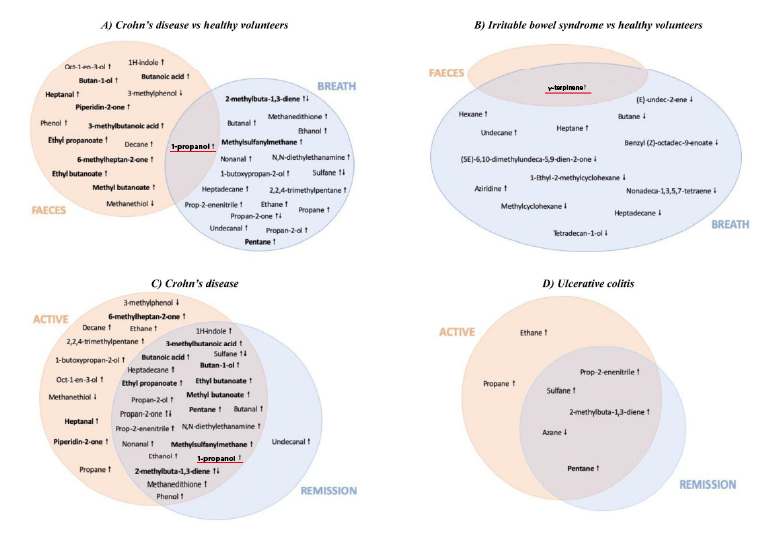
The authors concluded from the analysis two promising VOCs as single biomarkers: propan-1-ol (1-propanol) and 1-methyl-4-propan-2-ylcyclohexa-1,4-diene (γ-terpinene).
Four independently conducted studies showed increased 1-propanol from breath and/or fecal in subjects with Crohn’s disease (CD), a subtype of IBD, when compared to healthy volunteers (HVs) using GC-MS analysis. The compound, which implies inflammation, was described to decrease after CD subjects were treated. Besides the potential of CD diagnosis, 1-propanol was found increased in both breath and feces of CD subjects when compared to IBS-diarrhea (IBS-D, a subtype of IBS) subjects, suggesting its potential for differential diagnosis.
For diagnosing IBS, two studies sampling either breath or fecal with GC-MS analysis suggested γ-terpinene a promising VOC biomarker as it increased in IBS subjects when compared to HVs. It is unclear why the exogenous, plant-originated compound with anti-inflammatory properties increased in IBS subjects, but it could be a result of gut microbiome composition change – decrease of certain microbes that possess unknown enzymatic activities to metabolize γ-terpinene. Interestingly, γ-terpinene was also the only compound that showed differences between IBS subjects and HVs in the two studies.
Aside from single VOC biomarkers, combining a panel of VOCs in discriminative models is an advantageous approach to increase specificity, especially when conditions are highly variable and could be reflected upon inconsistent VOC findings. A total of 16 studies in the review described 17 models for discriminating disease subjects from HVs. ROC-AUCs ranged between 0.77-0.99 when discriminating CD subjects from HV in breath and feces. Models discriminating ulcerative colitis (UC, another subtype of IBD) subjects from HV yielded ROC-AUCs between 0.54-0.74, with lower numbers in fecal models. For IBS, the ROC-AUCs ranged between 0.44-0.92 in breath, fecal and urine samples.
Although only eight models discriminated between patient populations for differential diagnosis, the models showed better performance (ROC-AUC 0.7-0.97) than those comparing patients with HV. For disease monitoring, the results were rather contradictory. For instance, in one study, PCA analysis showed separation between active CD, inactive CD and HV subjects. However, the same could not be found between active UC, inactive UC and HV subjects. In another study, the analysis showed good separation between active and inactive UC subjects. However, the same could not be found between active IBD (pooling CD and UC subjects together) and inactive IBD. These results suggested the need for further research evaluation between active and inactive disease states.
As mentioned, IBS and IBD have been associated with the changes of the gut microbiome composition and stability. VOCs found in these diseases could be either produced by microbial metabolism or microbial-influenced metabolic processes in the body. For example, several discriminative compounds found in these studies are short chain fatty acids (SCFAs), which are products of bacterial fermentation with anti-inflammatory properties. One study by Smolinska et al. demonstrated the interplay between VOCs and gut microbiome for the first time through correlating exhaled breath VOCs and gut bacterial taxa in different CD disease states. Another study suggested a correlation of treatment-induced microbial changes with decreased fecal SCFAs in inactive CD subjects. These findings highlighted the need to take the microbial composition into account in future studies.
Conclusions
The review from Malderen et al. emphasized the importance of proper study design, considering appropriate controls and separating disease subtypes to reduce data variability. They suggested that combining VOC panels will also help increase the discrimination between different group comparisons. Most importantly, the interplay between VOCs and the gut microbiota should be taken into consideration, as correlation analysis will help decipher the complex interplay of microbial composition on baseline values in both patients and HVs.
At Owlstone Medical, we have the expertise to help customers with proper study designs and a well-developed platform for standardized breath sample collection and consistent analysis for identifying potential VOC biomarkers. Our Breath Biopsy VOC Atlas includes on-breath VOCs that are suggested products from gut microbial metabolism. Our recent webinar on ‘’Non-invasive Functional Assessment of the Microbiome from Exhaled Breath’ can help with your research in the gut microbiome field.
