Speed up clinical mass spec workflows for steroid analysis
Reduce LC run times and separate co-eluting steroid metabolites with ultraFAIMS
Rapid analysis of anabolic steroid metabolites in urine by combining field asymmetric waveform ion mobility spectrometry with liquid chromatography and mass spectrometry
Kayleigh L. Arthur, Matthew A. Turner, Alan David Brailsford, AndrewKicman, David A. Cowan, James C. Reynolds, and Colin S. Creaser
Detecting drugs and drug metabolites in urine and other clinical samples using mass spectrometry (MS) often requires long-winded chromatographic steps in order to separate structurally similar analytes. Even then, co-eluting isobaric species can greatly complicate the quantitative analysis of certain compounds. Speeding up analysis times for clinical samples is an important route to improving the efficiency of your clinical laboratory.
The analysis of anabolic androgenic steroids (AAS) and their metabolites in urine is an example of a common clinical lab process that could greatly benefit from reduced analysis time. AAS are abused by individuals aiming to improve performance in a range of sporting and bodybuilding disciplines. Amongst other effects, AAS stimulates muscle growth, and are also administered legitimately for a range of medical conditions.
This class of steroids includes naturally occurring compounds (e.g. testosterone) as well as structurally similar synthetic compounds. Detection of these steroids has become a focus of global anti-doping programs by sporting bodies, who have to analyse many thousands of samples in a tight timescale.
Conventionally, determination of endogenous AAS as either free drugs or their metabolites in urine uses GC-MS or less commonly LC-MS (gas chromatography-MS or liquid chromatography-MS).
The standard AAS analysis with GC-MS takes around 5 hours in total, this includes derivatization of the steroids prior to analysis and a long GC run time. LC-MS analysis may not require a derivatization step, but achieving adequate chromatographic separation of components still requires significant run time. The specificity and sensitivity of both techniques can be improved by using tandem mass spectrometry (MS/MS), however this does not allow the separation of isobaric interferences with similar retention times or the identification of structurally similar isobaric steroids.
To address the lengthy chromatographic run times and difficulty associated with the separation of these steroids and their metabolites, Arthur et al.1 investigated the inclusion of field asymmetric ion mobility spectrometry (FAIMS) as an extra separation step in an AAS analysis LC-MS workflow. The study used Owlstone Medical’s retrofittable ultraFAIMS instrument, which provides in-source separation of ions that is orthogonal to both LC and MS.

The authors found that it was possible to use a shorter LC run time, before separating co-eluting steroid metabolites using ultraFAIMS prior to MS. From sample introduction, this method took a total analysis time of just 8 minutes.
The steroids targeted are of interest to anti-doping laboratories, and included isobaric steroid sulfates: testosterone sulfate (TS), epitestosterone sulfate (ETS), and dehydroepiandrosterone sulfate (DHEAS); and two pairs of isobaric steroid glucuronides: testosterone glucuronide (TG), epitestosterone glucuronide (ETG) and androsterone glucuronide (ADG), and etiocholanolone glucuronide (ECG). These pairs of steroid glucuronides and trio of steroid sulfate metabolites are exact mass isomers which cannot be separated by even the highest resolution mass spectrometry.
The addition of ultraFAIMS allowed complete separation of species of interest in the urine matrix. Figure 2 summarises the separation achievable with LC-ultraFAIMS-MS compared to LC-MS and MS alone.
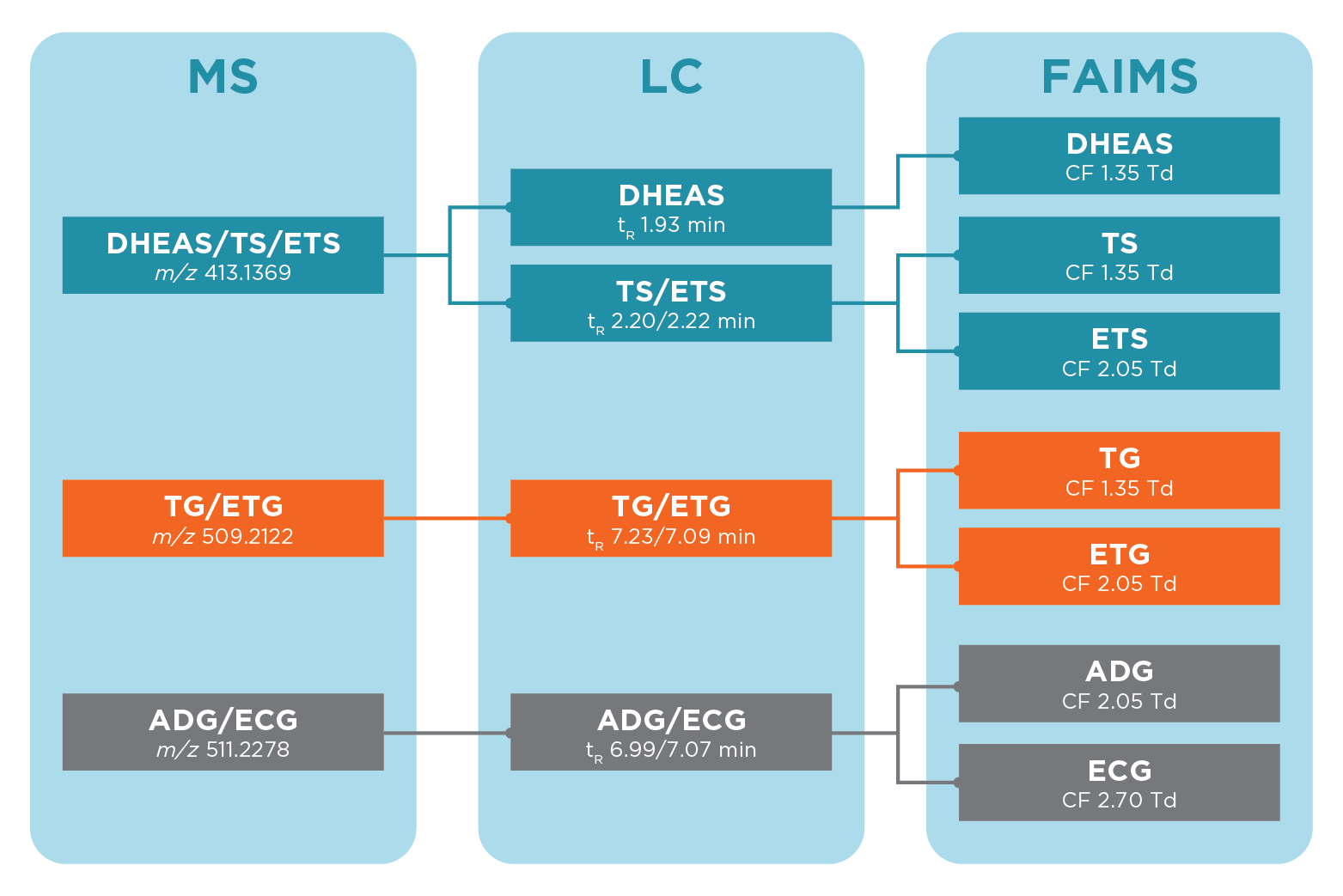
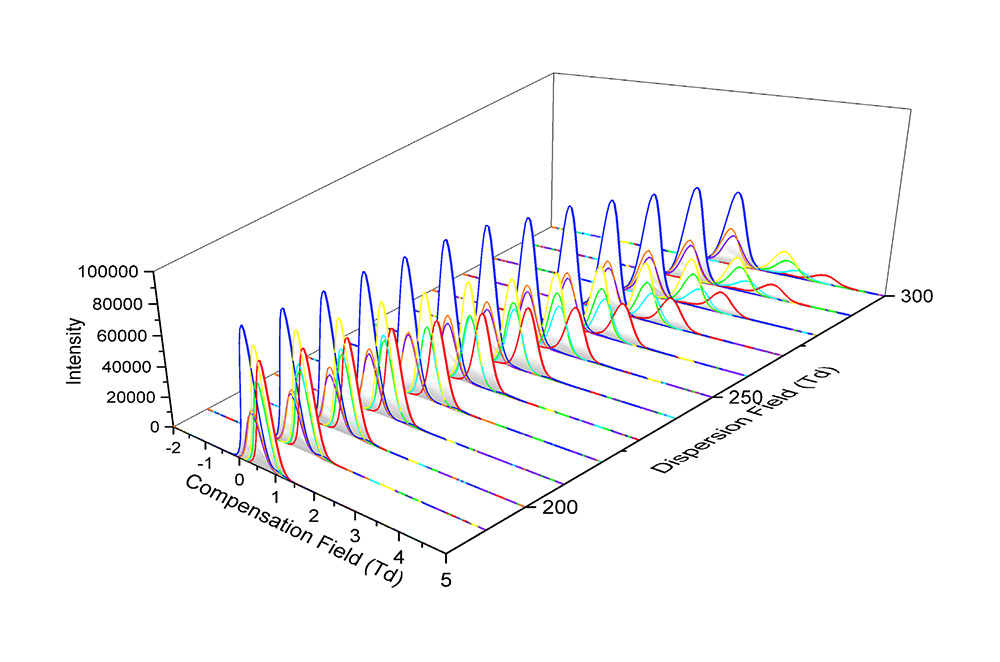
To achieve this complete separation of co-eluting species, it was necessary to sodiate the ions by a simple post column infusion of a sodium acetate solution. Figure 3 shows the separation achievable between the sodiated adducts across a range of FAIMS compensation field (CF) and dispersion field (DF) values.
As well as allowing separation of the metabolites, the addition of ultraFAIMS also reduced chemical noise from the urine matrix and improved signal to noise ratio (S:N). The authors note that the LC-ultraFAIMS-MS method showed good qualitative and quantitative responses for the steroid metabolites, and that it provided an additional identification tool using cluster patterns. They also suggested that higher sensitivity for the naturally low-abundant steroid metabolites could be achieved by combining the LC-ultraFAIMS-MS with tandem mass spectrometry, for example a triple quadrupole mass spectrometer.
Summary
The addition of ultraFAIMS to the LC-MS workflow improved the analysis of steroid metabolites in urine:
- Successfully separated co-eluting isobaric species
- Reduced chemical noise from the urine matrix and improved signal to noise ratio (S:N)
- Reduced total analysis time to 40 minutes (compared to 5 hours for the standard GC-MS workflow)
References
- Rapid analysis of anabolic steroid metabolites in urine by combining field asymmetric waveform ion mobility spectrometry with liquid chromatography and mass spectrometry, Arthur, K.L. et al., Analytical Chemistry, DOI: 10.1021/acs.analchem.7b00940.
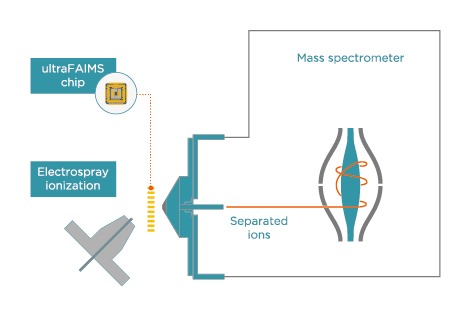
ultraFAIMS
Add ion mobility to existing mass spectrometers to provide in-source separation of ions
Retrofit to Thermo Scientific, Agilent, Waters and Bruker instruments


