Is breath acetone too ubiquitous to be a good biomarker of heart failure?
Evidence suggests acetone could be useful for both diagnosis and prognosis
| Publication information: Gouzi et al. Breath acetone concentration: too heterogeneous to constitute a diagnosis or prognosis biomarker in heart failure? A systematic review and meta-analysis J Breath Res. (2021) 6;16(1). DOI: 10.1088/1752-7163/ac356d
Disease Area: Heart failure (Cardiovascular and metabolic disease) Application: Diagnosis and prognosis Sample medium: Breath Analysis approach: Misc. (Review paper) Summary:
|
Heart Failure (HF), as a consequence of cardiovascular disease, is the main cause of hospitalization in the USA, Europe and Japan, with cases rising worldwide. Despite improved treatment in recent decades, HF maintains a strong association with poor long- and short-term outcomes for patients.
Biomarkers of cardiac disease have the potential to be of great benefit clinically. Currently a number of biomarkers of cardiovascular disease and heart function are already in clinical use, but accessing these biomarkers (such as plasma concentration of brain natriuretic peptide (BNP), cardiac catheterization and coronary angiography) is invasive and burdensome for the patient. Testing like this is also expensive for the healthcare provider. Considering the increasing prevalence and severity of HF, developing new less-invasive diagnostic, prognostic and monitoring biomarkers in HF patients would be of huge value.
A number of clinical studies have investigated breath biomarkers, specifically breath acetone, as one possible solution to bring positive change. Acetone is one of the most common metabolites produced by oxidative tissues, such as the heart, and alongside other ketones, has been shown to increase in concentration on the breath of both HF and diabetes patients. Gouzi et al.’s conducted a meta-analysis of all of the studies which have considered acetone as a potential biomarker of heart failure.
Inclusion Criteria
Considering papers published between 1991 and 2020 (as found on PubMed and Web of Science), Gouzi et al. included every paper that reported a measurement of acetone on the exhaled breath of adults HF patients compared to healthy controls, with a minimum of 15 subjects per study. Varied types of analytical methods were included, provided they were clearly detailed in the method. Studies without clear inclusion criteria for their HF patients were excluded. A final library of 18 qualifying studies was included in the meta-analysis.
Figure 1. Flow chart of the study selection
A total of 833 healthy controls (HC) and 1009 HF patients were analyzed, providing some quantitative and generalizable data regarding exhaled acetone (ExA) concentrations in adults worldwide. For these 18 studies, standardization factors (fasting), methodological issues (sensors, sampling and analytical methods), and HC definition altered the between study homogeneity. In general they observed that required fasting (even just overnight) prior to participation, reported higher concentrations of ExA than studies which measured ExA after uncontrolled diet. Demographic data of subjects was extracted from all studies and also found to affect acetone concentrations.
Conclusions
Forest plots showed a strong degree of heterogeneity in healthy controls ExA concentrations across the 18 studies analyzed. While the average of the pooled ExA concentrations in HF patients was 1.89 times higher than in healthy controls. This correlated well with the NYHA dyspnea scale and plasma BNP, established markers used with HF, highlighting ExA’s good diagnostic and prognostic accuracy in single studies.
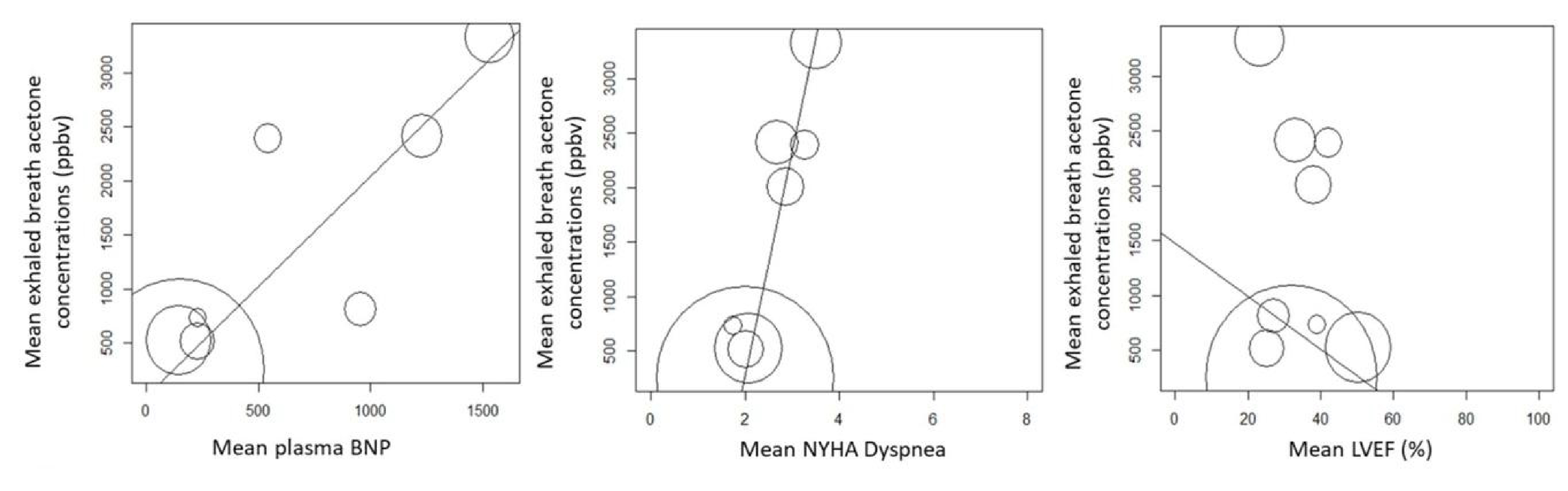
Figure 2: Relationships between mean exhaled breath acetone concentration and mean plasma BNP, mean NYHA dyspnea and mean LVEF.
Gouzi et al. were happy to conclude that ExA constitutes a promising diagnostic and prognostic biomarker in HF patients. They suggested future studies could move towards validation and, hopefully, regulatory approval by improving methods for standardizing ExA measurements (such as overnight fasting), and obtaining worldwide reference values in well-defined and fully characterized community-based healthy subjects, using a device with a good performance (i.e. GC-MS and/or other).
If you’d like to undertake further work that attempts to validate acetone as a biomarker for HF, or looks for further complementary biomarkers, we can help.
As interdisciplinary specialists in breath, we have created Breath Biopsy® OMNI® to be the most advanced solution for reliable standardized global breath biomarker analysis. And, when you choose to use our Breath Biopsy services, your investigation is further supported by our extensive study design, management, and data interpretation expertise.
