Breath Biopsy VOC Atlas®
About the VOC AtlasIn upcoming months we will be making specific VOC Atlas data/functionality accessible, through our new partnerships with the Bill & Melinda Gates Foundation and the FDA.
Sign up for access Read our Paper
Owlstone Medical has embarked on an exciting project to better understand the composition of human breath both in terms of understanding the diversity present in a healthy population, and the differences in different disease states. Through this work, we have developed the Breath Biopsy VOC Atlas®, a catalog of identified and quantified volatile organic compounds (VOCs) found in exhaled breath. The Atlas also provides insight and scientific context to identified compounds to enable the confident selection of candidate biomarkers for a variety of diseases such as asthma, chronic kidney disease, COPD, liver cirrhosis, and more.
VOCs can be utilized as non-invasive biomarkers
VOCs are a group of compounds that can arise from endogenous underlying metabolism such as lipid peroxidation products, or exogenous sources such as microbiome-originating metabolism or the diet. These VOCs are detectable in many bodily fluids including breath, as VOCs can diffuse from their point of origin and travel through the bloodstream to the alveolar membrane, where they readily volatilize into the air in the lungs. Due to the link between VOCs and their generation from, or interaction with the physiological processes in the body, specific VOCs could provide significant benefits as non-invasive biomarkers, detectable in the breath.
The Breath Biopsy® VOC Atlas
We have developed the Breath Biopsy VOC Atlas as a catalog of VOCs that are commonly found in exhaled breath. The initial cohort data entered into the Atlas was specifically structured to include subjects with no specific pathophysiologies across a variety of ages and ethnic backgrounds to assess the composition, and “normal” variation in the composition of human breath. Since that initial cohort, we have increased the capacity of the Atlas to also contain disease cohorts and a broader view of the VOC composition of breath under a range of contexts to provide a more detailed understanding of human breath as a platform for biomarker analysis. The Atlas encompasses data from a variety of disease cohorts, including colorectal cancer, chronic kidney disease, COPD, and liver cirrhosis.
The data from the initial healthy human cohort can be used as a proxy for the normal variation in the population, and serve as a useful baseline to streamline breath biomarker discovery and validation, for example, to identify VOC targets that are likely to be impacted by the disease process of interest. Our Breath Biopsy VOC Atlas is a rich repository of breath-related volatile organic compounds (VOCs). The database, search interface, and analytics tools present high-confidence VOCs alongside their clinical, chemical, and biological context.
The integrity of the Breath Biopsy VOC Atlas relies on stringent criteria for four key aspects:
- Robust breath collection and analytical procedures
- Distinguishing breath VOCs (on-breath) from background contaminants
- Confirming the chemical identity of VOCs
- Accurate Quantification
There are currently over 200 VOCs from a broad range of chemical classes in the Atlas, but this is expanding rapidly as we extend our technical capabilities and conduct further studies.
We recently published a paper in Metabolomics, “High-quality identification of volatile organic compounds (VOCs) originating from breath”. This paper builds the foundations of our soon-to-release Breath Biopsy VOC Atlas®,which you can now sign up for access.
1. Robust breath collection and analytical procedures
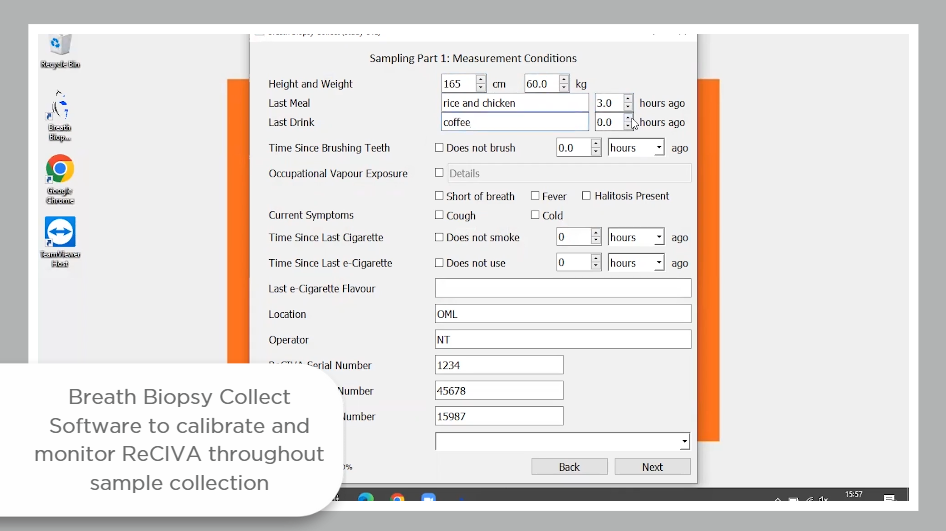
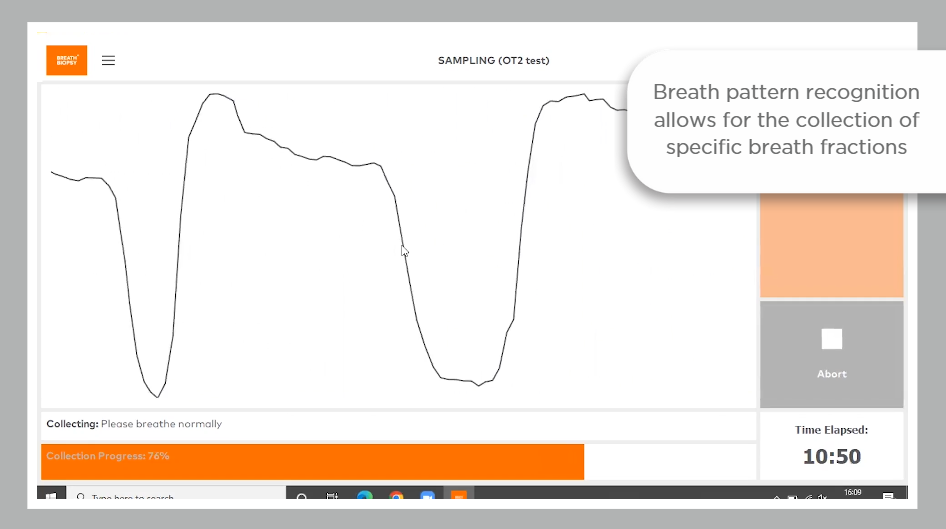
Due to historic challenges of standardization and repeatability in the breath research field, VOCs entered into the VOC Atlas have all been acquired from breath samples collected using Breath Biopsy technology, and analyzed using the Breath Biopsy OMNI platform.
The ReCIVA pre-concentrates breath samples on adsorbent tubes, enabling a larger collection of air volume and offering the potential for greater sensitivity in detecting low-abundance compounds. The pre-concentrated breath samples remain stable for longer periods of time at room temperature, and the small size allows for easier transportation and storage. This circumvents some of the challenges of sampling efficiency and storage stability associated with other, lower-cost options for breath collection. The CASPER provides a source of filtered clean air to industry standards for inhaled air, and the Collect software allows for the collection of the same volume of breath from every participant.
Read our Breath Biopsy Technology whitepaper for more information.
The OMNI platform incorporates analysis of breath samples using gold-standard high-resolution accurate mass TD-GC-MS, followed by robust statistical approaches. Thermal desorption (TD) as a means of sample introduction allows for the preconcentration of the breath sample on a focusing trap, enabling heightened sensitivity for trace-level VOC detection. Confidence in results is maximized by precise calibration and QC procedures, both for individual samples and across the analytical pipeline.
Read our OMNI whitepaper for more information
2. Distinguishing breath VOCs (“on-breath”) from background contaminants
One of the strengths of on-breath compounds entered into the VOC Atlas in each study cohort is that they have undergone robust analytical comparison to background samples. This is designed to discriminate VOCs in the breath from background contaminant VOCs that originate from the environment, or from breath sampling equipment that have been inhaled immediately prior to breath sampling.
Currently, background samples are collected immediately before each breath sample using the same equipment and procedures to reflect the VOC background at the time of measurement. The background samples are also collected from the entire equipment flow path of air to capture all possible sources of VOC background in the sampling process. VOCs are only identified if they meet pre-defined metrics used to compare breath to representative system background samples. There are three metrics in use at Owlstone Medical:
- The standard deviation (SD) metric: The mean average signal for each VOC is calculated across all system background samples within the cohort. A VOC feature is considered on-breath if the signal exceeds the mean of the system background signal plus 3 SDs in at least 50% of the breath samples from the cohort. If values are observed for fewer than 4 system background samples, then by this metric a VOC is automatically on-breath.
- The paired T-test approach metric: VOCs in each breath sample are compared to the corresponding VOC in the paired system background sample. A feature is regarded as on-breath if it is associated with a fold difference ≥ 2 and paired t-test one-tailed p-value ≤ 0.05.
- The Receiver Operating characteristic area under the curve [ROC-AUC] metric: Each VOC is used to generate a ROC-AUC value for the classification of samples as either breath or system background. A feature is considered to be on-breath if the fold difference between breath and background is > 1, and the calculated ROC-AUC value is ≥ 0.8.
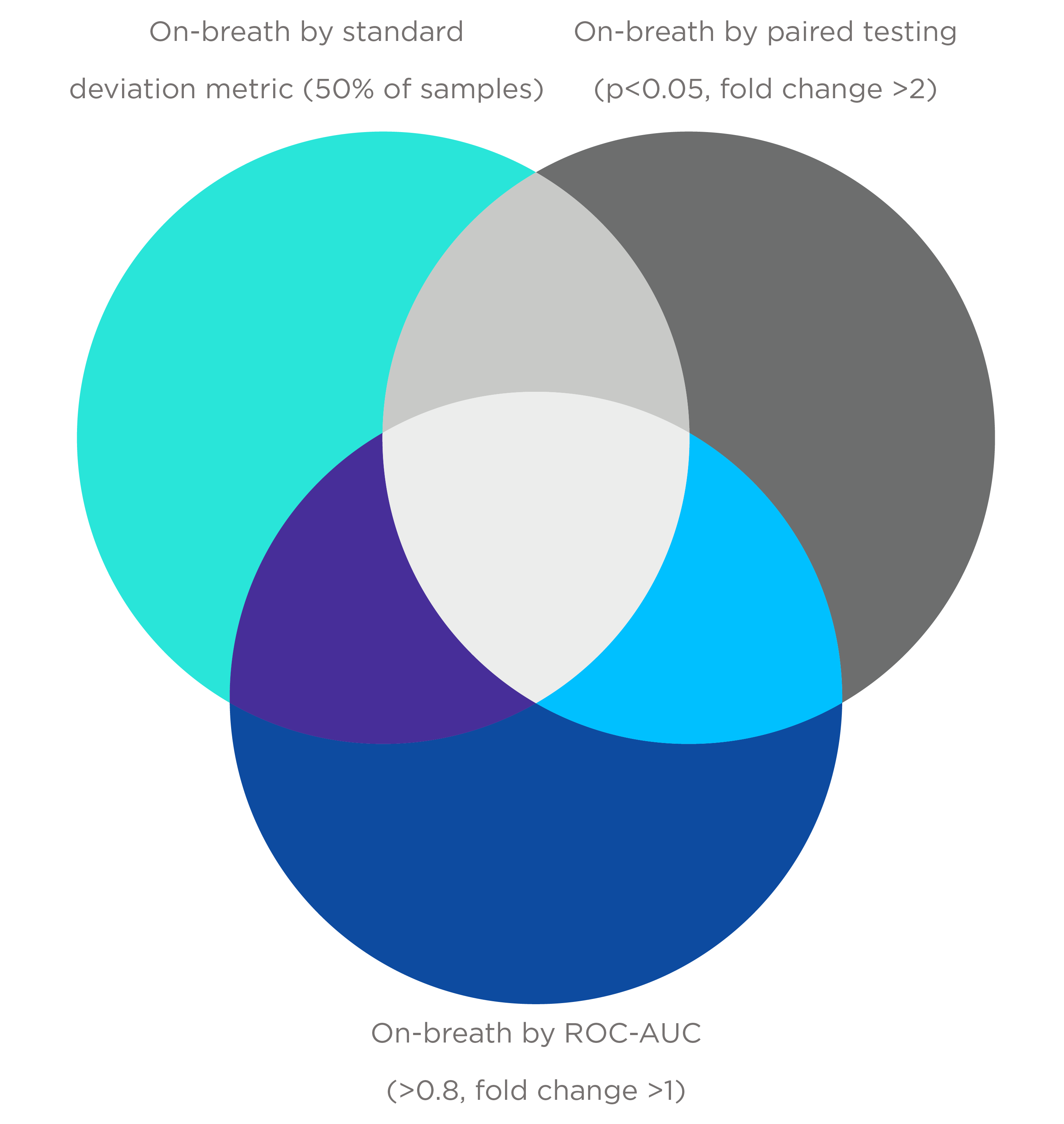
Each metric can classify distinct on-breath VOCs, as no single method may be universally appropriate and different levels of stringency for on-breath assignment can be used based on study design.
3. Confirming the chemical identity of VOCs
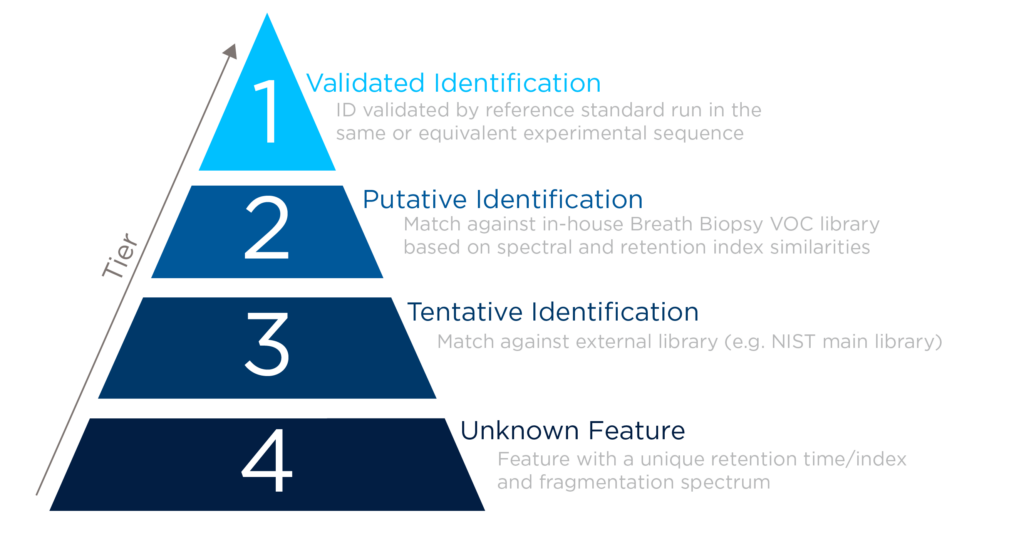
Stringent processes are applied to support high-confidence compound identification; reference standards are run on the same analytical method, high-resolution fragmentation spectra are compared within tolerance criteria, and retention indices are compared against an alkane ladder. Retention indexing is particularly valuable for preventing misidentification of VOCs due to intrachemical class spectral similarity. High specificity is provided by accurately determining molecular formulas and distinguishing between different VOCs with similar masses.
VOCs entered in the VOC Atlas have typically achieved a tier 1 level of confidence, and all are labeled according to the MSI standard for compound identification.
4. Accurate Quantification
Accurate quantification of the abundance of on-breath VOCs is essential to robust biomarker discovery and validation. To quantify the concentration of VOCs, a calibration curve of the target at a suitably tailored concentration range is run per compound to encapsulate levels observed in breath, alongside the breath samples where possible. This is then used to determine the ng on sample value based on the peak area signal. This value can then be converted into breath concentration by dividing the ng value by the total volume of breath collected.
We look forward to sharing additional classes of molecules, and their clinical relevance, as we continue to better understand the diversity of molecules in the breath. For more information on our VOC Atlas and the compounds included, please get in touch, or to gain access to the VOC Atlas, please fill out the form.
Catch up on the presentations from the Breath Biopsy Conference 2024