Breath VOC biomarkers for Cystic Fibrosis – steps towards the development of bacterial infection biomarkers for clinical use
We have combined our internal data with VOCs identified across the literature to date (June 2024) and outline important next steps for selecting promising biomarkers for translation into clinical testing in the future.
| Disease Area: Cystic Fibrosis
Application: Biomarker Development Sample medium: Breath Analysis approach: GC-MS, SPME GC-MS, PTR-TOF-MS, quantum cascade laser-based spectroscopy, GCxGC-TOF-MS, GCxGC-MS Summary:
|
Introduction
Cystic Fibrosis (CF) is a genetic disease affecting over 80,000 people worldwide. About 75% of CF patients are diagnosed by age two. Since there is no cure, routine monitoring and assessment are crucial for long-term management. CF patients have impaired fluid transport across the epithelial cell membranes due to a CFTR (transmembrane conductance regulator) gene mutation. This leads to thick and viscous mucus buildup at the cell surface, creating an environment prone to colonization and infection by opportunistic pathogens, such as Staphylococcus aureus and Pseudomonas aeruginosa. Despite medications that aid mucus clearance, a sudden worsening of clinical conditions, namely, pulmonary exacerbation, remains the most significant cause of morbidity. This is likely related to the complex relationship between bacterial infection and host defense, which affects sputum production and airflow obstruction. Detecting bacterial colonization or early infection in CF patients is vital for early treatment, which could help minimize the impact on lung function. Monitoring treatment responses will also help customize the duration of antibiotic use, avoiding the development of antibiotic resistance over time.
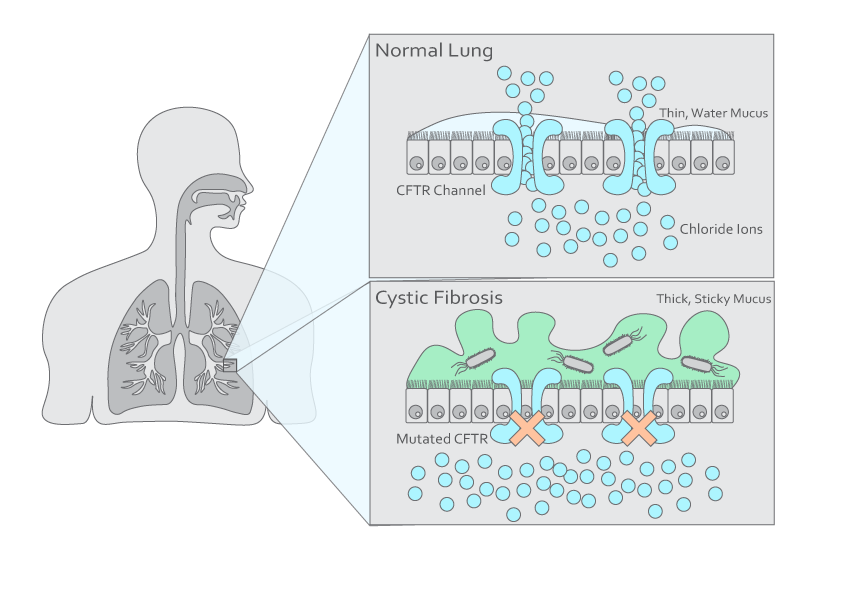
Figure 1. Illustration of a normal lung and a cystic fibrosis lung.
Breath tests are an ideal tool for detecting bacterial colonization/infection and monitoring treatment in CF patients due to the direct contact breath has with the respiratory tract, and the non-invasive sampling process, which offers more comfort to patients. This is particularly important for CF patients because they require frequent monitoring, and the current standard method of sputum sampling can be unpleasant for some patients. Exhaled breath contains volatile organic compounds (VOCs) which can reflect physiological changes in the body. VOCs are potential biomarkers with two origins (endogenous and exogenous), which can be selected strategically depending on the disease and purpose of the test. In CF, both endogenous and exogenous VOCs can be used as biomarkers. Endogenous VOCs indicative of airway inflammation are a common pathophysiology resulting from oxidative stress, while exogenous VOCs produced by bacteria can offer early detection of infection or colonization. Breath VOC biomarkers could potentially be developed into a home-based test, saving patients from regular clinic visits and reducing pathogen exposure.
Dr. Kavita Jeerage from the National Institute of Standards and Technology (NIST) recently presented at the Breath Biopsy Conference 2024, on ‘Potential Uses of Breath Surrogates for the Development and Deployment of New Infectious Disease Biomarkers’. You can watch Dr. Jeerage’s talk on-demand, as well as the rest of the talks from the conference here.
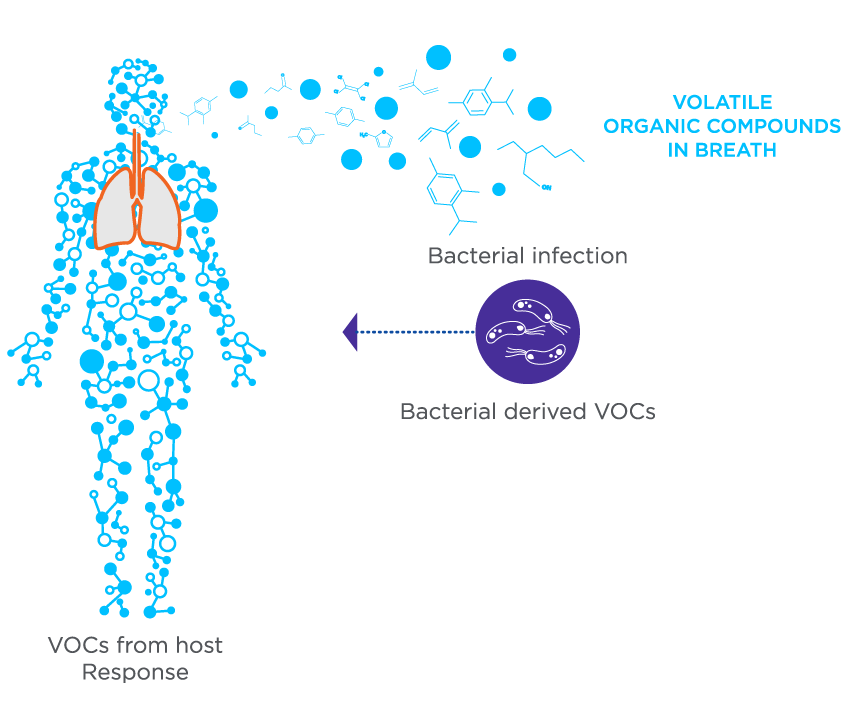
Figure 2. The VOCs in breath during an infection can originate from bacterial metabolism as well as the host’s response to infection.
Results
The Breath Biopsy VOC Atlas is a database we developed to include detectable exhaled breath VOCs from our analytical platform. It also aggregates compounds reported in the literature across different disease areas, with URLs linking to original publications for researchers’ convenience. These features benefit users by providing easy access to information and allowing them to select compounds of interest. Within the CF disease area, 19 unique VOCs combined are reported from the literature and our internal data in the VOC Atlas [1, 3-15]. The VOCs can be chemically classified into ketones (5), alkanes (2), aldehydes (3), carboxylic acids (3), alcohol (1), fatty acid ester (1), terpene (1), sulfur-containing compound (1), diacetyl (1), and toluene (1). These include pathogen-produced VOCs like 2-butanone and 2-nonanone, and indicators of inflammation like pentane. However, 2,4-Dimethylheptane is not present in the VOC Atlas currently. The 19 compounds in the VOC Atlas are commonly present on-breath in humans, with their identities confirmed by standards. Only 53% (10 compounds) reported in CF literature have identities confirmed by authors, therefore, whether all 19 compounds remain potential biomarkers, despite them being on-breath with confirmed identities will require additional research.
Discussion
Studies to distinguish breath profiles between CF patients and controls, as well as identify potential biomarkers, have been ongoing for the last two decades. While these studies show promising results, no biomarkers have been developed for clinical testing of bacterial infection in CF patients. This challenge is partly due to the difficulty in replicating VOC findings across studies, primarily because of the lack of standardization in breath sampling (e.g. fasting hours, background air consideration) and compound confirmation with reference standards. A study published in 2022 selected 56 VOCs upon conducting literature review and performed a targeted analysis using breath samples of 53 CF subjects (both with and without P. aeruginosa infection)[1]. Only 13 out of the 56 VOCs were detected, highlighting the importance of standardization. Additionally, the study found that the abundance of compounds in breath differed by age group. In children (n=25), the levels of 2-butanone, ethyl acetate, and 2,4-dimethylheptane significantly differed between those with and without P. aeruginosa infection. None of the 13 detected compounds showed significant differences in adults with or without P. aeruginosa infection. It is likely that these compounds originate from both endogenous and bacterial metabolism, and perhaps the endogenously produced levels in different adults are more varied in general than in different children, therefore masking the statistical differences between subjects with or without infection. This suggests that with the goal of testing patients of all ages, an ideal biomarker selected for development should originate from bacterial metabolism. If the biomarker for development is also produced endogenously, which can be more common the case, the levels produced should have less inter-subject variability. Additionally, it also emphasizes the necessity to build breath profiles with quantified VOC levels, and identify the abundance range that may differ by age and/or other factors. Of the 13 compounds detected in the 2022 study, only four VOCs (2-pentanone, 2-butanone, ethyl acetate, and toluene) were common between the 19 CF-associated VOCs that appear in our VOC Atlas.
In the case of bacterial infection, the absolute quantification of VOCs in breath will help to distinguish between endogenous and exogenous breath VOCs, especially as some can originate partially from both endogenous and exogenous origin. Identifying VOCs from bacterial metabolism and detecting them in exhaled breath alongside absolute quantification will advance biomarker development into clinical settings. In vitro studies have suggested both direct and indirect evidence of several compounds that can be produced by P. aeruginosa, including 2-butanone and 2-nonanone [2, 3]. Detecting these pathogen-produced compounds in exhaled breath and establishing concentration changes between CF subjects with and without infection (ideally a longitudinal study where subjects compare breath profile changes with themselves) are crucial steps to facilitate the translation of biomarkers into clinical settings.
Regardless, VOC characterization, identity confirmation, and quantification are key steps necessary to facilitate breath biomarker research and development into clinical use. The VOC Atlas can help researchers in the CF disease area to identify high-confidence on-breath VOCs and their concentration ranges in patients with and without specific pathogen infections, progressing the development of biomarkers for clinical use and application of point-of-care devices to easily monitor infection.
At Owlstone Medical, our Breath Biopsy OMNI® service provides standardized breath sampling and analysis to help researchers to identify novel biomarkers and proceed with further validation. The use of Breath Biopsy VOC Atlas can be incorporated based on research needs. To find out more information about breath biomarkers for cystic fibrosis, and other potential research studied involving the use of VOCs, please contact us.
References
- Kos, R., et al., Targeted exhaled breath analysis for detection of Pseudomonas aeruginosa in cystic fibrosis patients. J Cyst Fibros, 2022. 21(1): p. e28-e34. DOI: 10.1016/j.jcf.2021.04.015
- Zscheppank, C., et al., Investigation of volatile metabolites during growth of Escherichia coli and Pseudomonas aeruginosa by needle trap-GC-MS. Anal Bioanal Chem, 2014. 406(26): p. 6617-28.
- Purcaro, G., et al., Volatile fingerprinting of Pseudomonas aeruginosa and respiratory syncytial virus infection in an in vitro cystic fibrosis co-infection model. J Breath Res, 2018. 12(4): p. 046001. DOI: 10.1007/s00216-014-8111-2
- Mustafina, M., et al., Exhaled breath analysis in adult patients with cystic fibrosis by real-time proton mass spectrometry. Clin Chim Acta, 2024. 560: p. 119733. DOI: 10.1016/j.cca.2024.119733
- Vernocchi, P., et al., Gut microbiota signatures in cystic fibrosis: Loss of host CFTR function drives the microbiota enterophenotype. PLoS One, 2018. 13(12): p. e0208171. DOI: 10.1371/journal.pone.0208171
- Nasir, M., et al., Volatile molecules from bronchoalveolar lavage fluid can ‘rule-in’ Pseudomonas aeruginosa and ‘rule-out’ Staphylococcus aureus infections in cystic fibrosis patients. Sci Rep, 2018. 8(1): p. 826. DOI: 10.1038/s41598-017-18491-8
- Phan, J., et al., Stable isotope profiles reveal active production of VOCs from human-associated microbes. J Breath Res, 2017. 11(1): p. 017101. DOI: 10.1088/1752-7163/aa5833
- Bos, L.D., et al., Bacteria in the airways of patients with cystic fibrosis are genetically capable of producing VOCs in breath. J Breath Res, 2016. 10(4): p. 047103. DOI: 10.1088/1752-7163/10/4/047103
- Neerincx, A.H., et al., Detection of Staphylococcus aureus in cystic fibrosis patients using breath VOC profiles. J Breath Res, 2016. 10(4): p. 046014. DOI: 10.1088/1752-7155/10/4/046014
- Dryahina, K., et al., Differentiation of pulmonary bacterial pathogens in cystic fibrosis by volatile metabolites emitted by their in vitro cultures: Pseudomonas aeruginosa, Staphylococcus aureus, Stenotrophomonas maltophilia and the Burkholderia cepacia complex. J Breath Res, 2016. 10(3): p. 037102. DOI: 10.1088/1752-7155/10/3/037102
- van Mastrigt, E., et al., Exhaled breath profiling using broadband quantum cascade laser-based spectroscopy in healthy children and children with asthma and cystic fibrosis. J Breath Res, 2016. 10(2): p. 026003. DOI: 10.1088/1752-7155/10/2/026003
- Whiteson, K.L., et al., Breath gas metabolites and bacterial metagenomes from cystic fibrosis airways indicate active pH neutral 2,3-butanedione fermentation. ISME J, 2014. 8(6): p. 1247-58. DOI: 10.1038/ismej.2013.229
- Savelev, S.U., et al., Volatile biomarkers of Pseudomonas aeruginosa in cystic fibrosis and noncystic fibrosis bronchiectasis. Lett Appl Microbiol, 2011. 52(6): p. 610-3. DOI: 10.1111/j.1472-765X.2011.03049.x
- Barker, M., et al., Volatile organic compounds in the exhaled breath of young patients with cystic fibrosis. Eur Respir J, 2006. 27(5): p. 929-36. DOI: 10.1183/09031936.06.00085105
- McGrath, L.T., et al., Breath isoprene during acute respiratory exacerbation in cystic fibrosis. Eur Respir J, 2000. 16(6): p. 1065-9. DOI: 10.1034/j.1399-3003.2000.16f08.x
