Navigating the Breath Biopsy® VOC Atlas®- A Case Study on Selecting Potential Compounds for Biomarker Development in Tuberculosis
As of August 2024, we have collated 192 VOCs from 25 research papers on Tuberculosis. In this case study we demonstrate how to use the information provided in our VOC Atlas to select potential compounds for biomarker development, using TB as an example.
| Disease Area: Tuberculosis (TB)
Application: Biomarker Development Sample medium: Breath, Skin, Urine, Feces, Cell culture Analysis approach: GC-MS, GC-qMS, GCXGC-TOF-MS, PTR-MS, HPPI-TOF-MS, GC-TOF-MS, SIFT-MS, TD-GC-MS, GC-EIMS, GC-IMS Summary:
|
The need for TB tests that can be rapid and inexpensive
Tuberculosis (TB) is an airborne infectious disease caused by Mycobacterium Tuberculosis (M. tb).
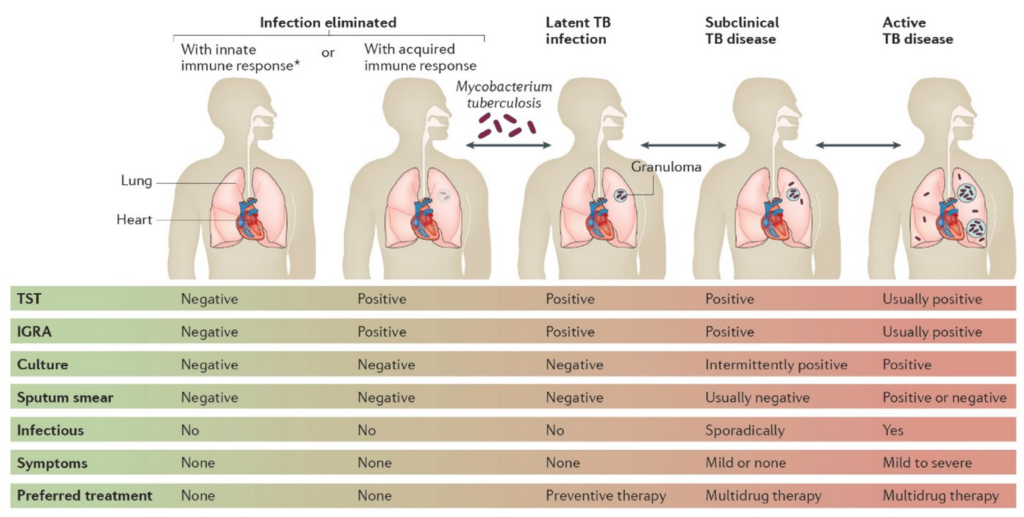
Figure 1 – A graphic taken from Pai et al.,[1] summarizing the different tests available for diagnosing and monitoring different stages of TB.
Roughly 25% of the global population is infected with M.tb, though the mycobacteria can remain dormant (latent TB) within the granulomas for years [1]. Around 10% of those infected may develop active TB, accounting for 1.5 million deaths every year. Early diagnosis and effective antibiotic treatment are crucial to prevent deaths and the spread of the disease. Currently, common TB tests include skin (Mantoux tuberculin skin test, TST) and blood (interferon gamma release assay, IGRA) tests [2] . TST, while simple to perform and at a low cost, requires two or more patient visits and trained personnel to administer and interpret results. IGRA, on the other hand, requires only patient visits but can be more expensive than TST. Both tests cannot distinguish active from latent TB, therefore further tests are required, costing more time and expense.
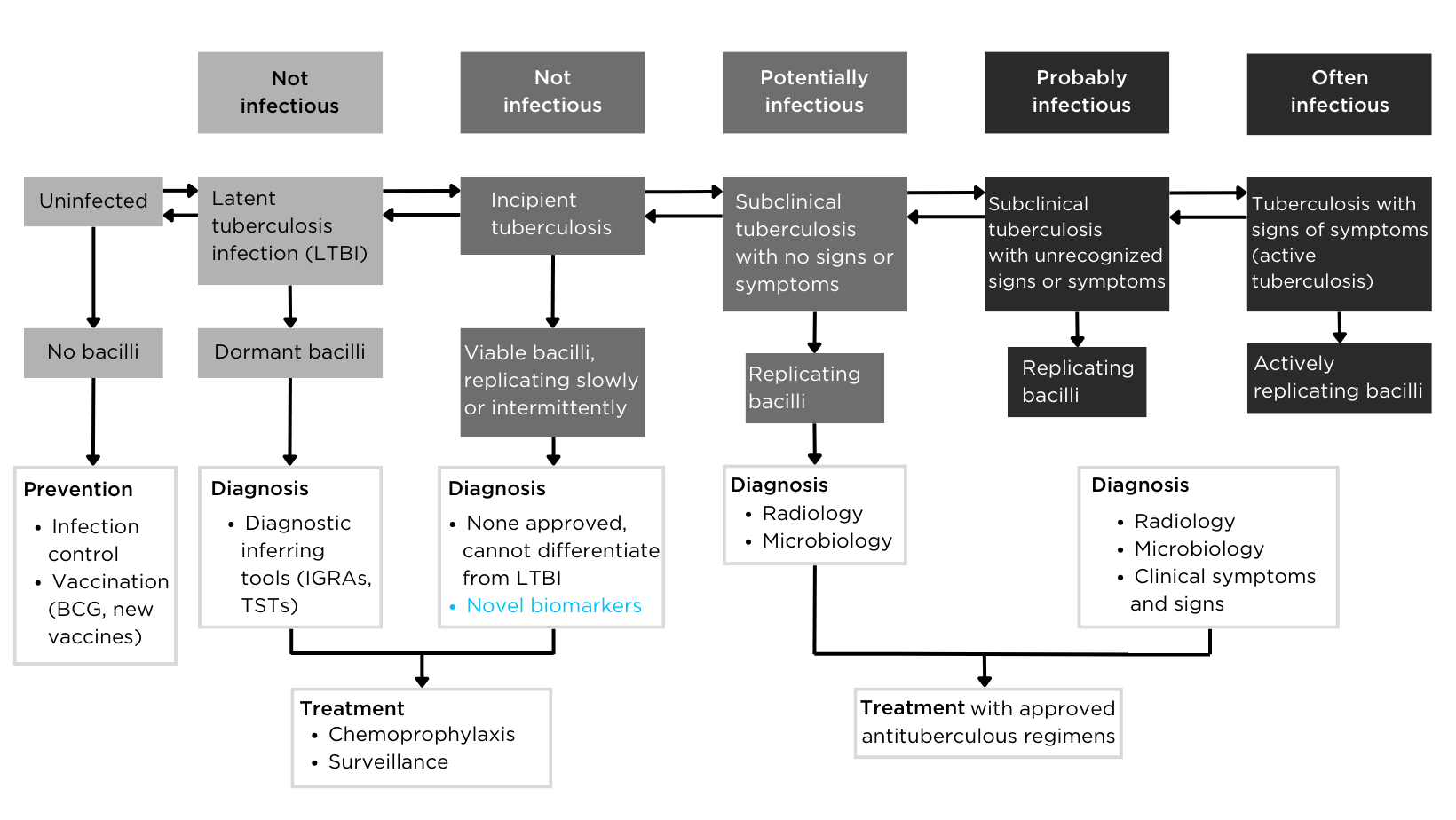
Figure 2 – The definition of tuberculosis infection based on the spectrum of tuberculosis disease.
Breath tests are an ideal alternative tool for TB detection due to their non-invasiveness, potential low cost, and rapid (real-time) results. Exhaled breath contains volatile organic compounds (VOCs) that reflect physiological changes in the body. Potential VOC biomarkers may originate from the mycobacteria (exogenous VOCs), or from the human body’s response to mycobacteria infection (endogenous VOCs). A recent study demonstrated that breath VOCs could distinguish latent TB from active TB and healthy controls with 80.0% sensitivity and 80.8% specificity [3]. While the tentatively identified VOCs require confirmation, the study shows the potential of breath tests for early disease detection.
Dr. Kavita Jeerage from the National Institute of Standards and Technology (NIST) recently presented at the Breath Biopsy Conference 2024, on ‘Potential Uses of Breath Surrogates for the Development and Deployment of New Infectious Disease Biomarkers’. You can watch Dr. Jeerage’s talk on-demand, as well as the rest of the talks from the conference here.
The VOC Atlas is a resource for researchers to select potential biomarkers for development
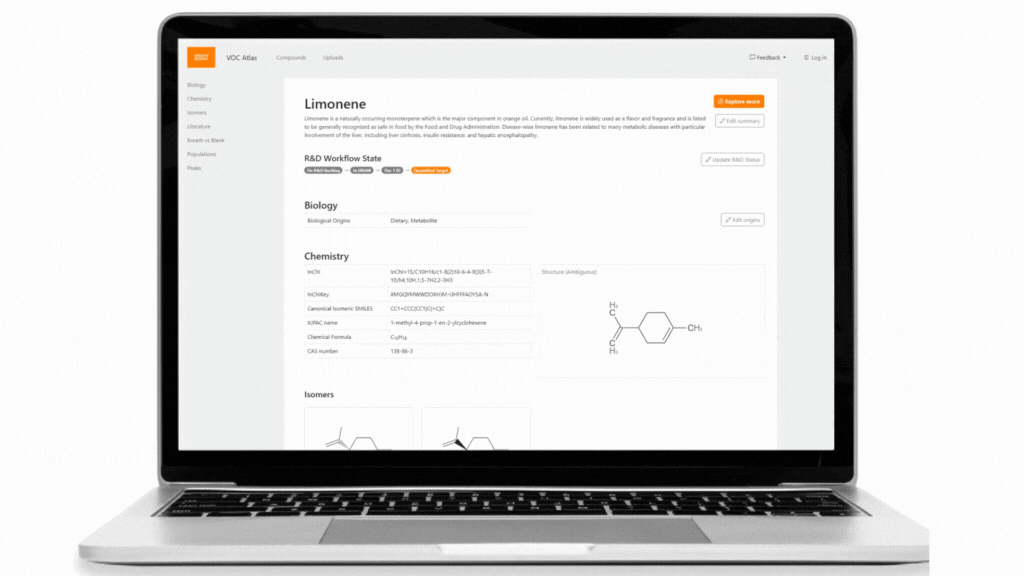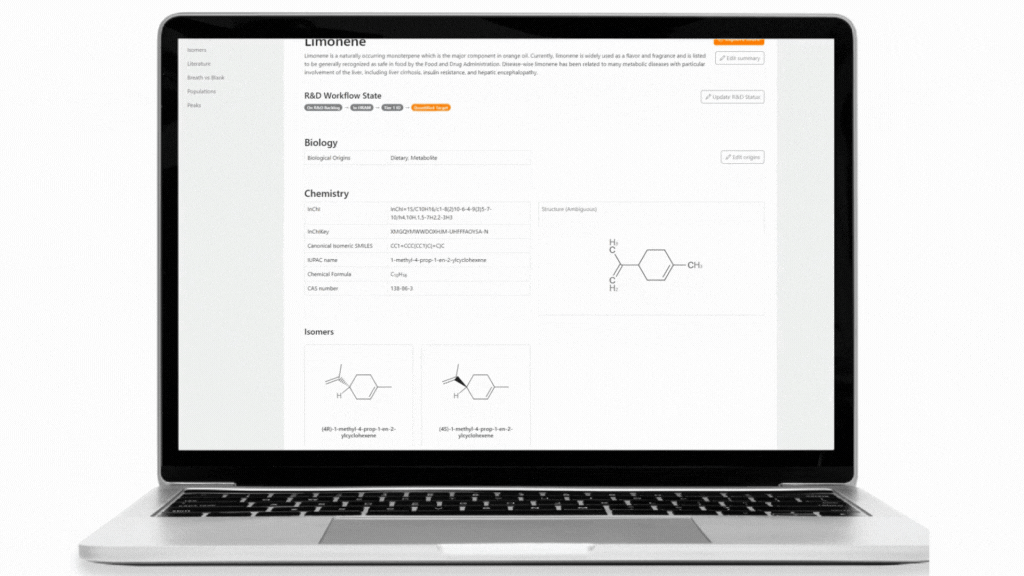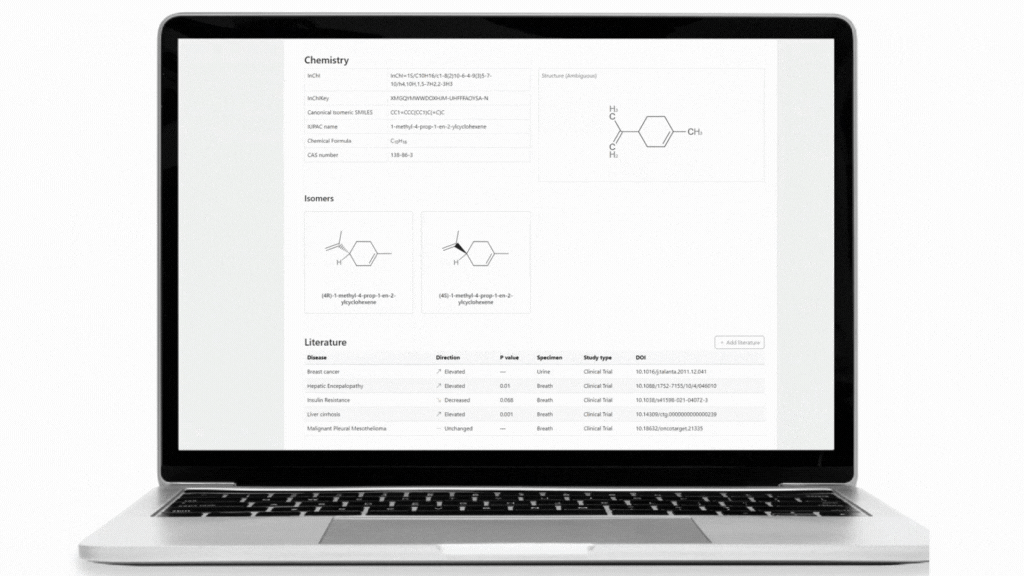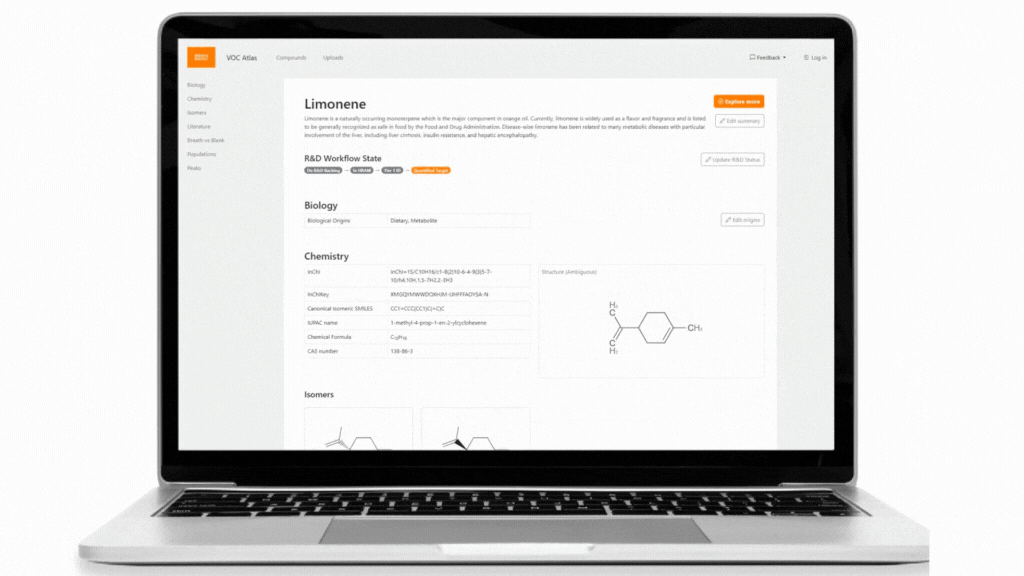
The Breath Biopsy VOC Atlas is a database we developed to facilitate researchers in their studies. It includes detectable exhaled breath VOCs at baseline (determined through a healthy cohort) from our analytical platform with confirmed identities by reference standards. Additionally, we developed specific metrics to differentiate compounds that are considered on-breath, meaning they are detected in exhaled breath with certain abundance differences from blank samples. The on-breath status of certain compounds, such as those associated with immune response, or those with exogenous origins in infectious diseases, are most likely to change as we add data from new studies with disease cohorts to the database. The VOC Atlas also aggregates compounds reported in the literature across different disease areas, with URLs linking to original publications. These features allow users to easily access information and select compounds of interest within specific disease areas to focus on.
Examples of potential compounds in the VOC Atlas for TB biomarker development
Within the TB disease area, 73 of the 192 VOCs from our literature review can be found in the VOC Atlas. Of these 73 VOCs, 23 are identified as on-breath from our developed metrics, and 9 of these on-breath compounds have appeared in more than one study.
Although not classified as on-breath in the VOC Atlas, 2-Butanone is an interesting candidate due to its abundance change in three studies: one in vitro (bacterial culture) study and two human breath studies [3, 4, 8]. Despite being tentatively identified in the human breath studies, the confirmed identity from the in vitro study indicates that 2-butanone originates from mycobacterium, which is why it is not classified as on-breath in the current VOC Atlas. A targeted analysis of 2-butanone in exhaled breath of TB-infected versus non-infected subjects could validate the compound and its transferability from in vitro to clinical studies. If validated, 2-butanone can potentially be a marker to distinguish between latent TB and active TB if the abundance level differs. Absolute quantification of 2-butanone levels in subjects with latent and active TB, as well as correlating with M.tb load, can facilitate the development of this compound as a biomarker.
Besides 2-butanone, an additional 11 compounds (tridecane, decane, 3-methylbutanal, 3-heptanone, nonanal, hexanal, heptanal, 2-ethyl-1-hexanol, o-cymene, camphene, and camphor) are found in at least two TB studies but do not appear as on-breath in the VOC Atlas. More than half of these compounds are being classified as alkanes and aldehydes, which are often reported in breath studies and can indicate inflammation. Since breath studies often report compounds with tentative identities, these compounds require validation. While they may not differentiate between latent and active TB due to immune responses in latent TB, they still hold potential as markers to distinguish infected from non-infected subjects, allowing for the development of non-invasive, inexpensive, and rapid breath tests for TB.
Some compounds, while reported in several TB studies and appearing on-breath in our VOC Atlas, should be selected with caution. For example, toluene was reported most frequently (6 times, all tentatively identified) in the collated literature, including human breath and skin, and animal breath, feces, and skin [3, 4, 15, 21-23]. Toluene is an exogenous compound from environmental exposures, often associated with smoking, thus its relevance to TB may be low. While its abundance change might result from the disruption of xenobiotic metabolism by bacteria, further evidence from TB research is needed to support this hypothesis. Similarly, methyl acetate is another compound that showed abundance differences in at least two TB infection animal studies [22, 27]. While these studies along with the on-breath status and identity confidence in the VOC Atlas suggest it may be a biomarker of interest, methyl acetate is also a compound related to environmental exposure. Whether methyl acetate differs between the exhaled breath of TB-infected and non-infected humans still requires further investigation to assess its disease relevance.
Some compounds in the literature have confirmed identities by the authors but are not yet included in the VOC Atlas. These compounds include methyl nicotinate, methyl p-anisate, methyl phenyl-acetate, o- phenylanisole [14], and 4-methyloctane [25].
Conclusion
As demonstrated above, researchers can select VOCs to focus on based on their needs and the information provided by the VOC Atlas. In the case of TB, biomarkers that can distinguish TB infection from other respiratory infections or differentiate active TB from latent TB will be promising candidates for further clinical test development. Currently, antibiotic resistance is a major challenge in TB treatment. Identifying VOC biomarkers that can be used to monitor treatment or indicate treatment response will also be very useful for clinical applications.
The VOC Atlas database is frequently updated with new compounds that have confirmed identities and assessed on-breath status. Researchers can search for compounds by disease area of interest or explore diseases associated with specific compounds. If you would like to experience using the VOC Atlas firsthand, please sign up to receive access as soon as it is live. Additionally, please feel free to contact us if you want to learn more about how we can assist with VOC biomarker discovery and development.
References
- Pai M, Behr MA, Dowdy D, Dheda K, Divangahi M, Boehme CC, et al. Tuberculosis. Nat Rev Dis Primers. 2016 Oct 27;2(1):1–23. doi: 10.1038/nrdp.2016.76
- CDC. Tuberculosis (TB). 2024 [cited 2024 Aug 23]. Testing for Tuberculosis. Available from: https://www.cdc.gov/tb/testing/index.html
- Fu L, Feng Y, Ren T, Yang M, Yang Q, Lin Y, et al. Detecting latent tuberculosis infection with a breath test using mass spectrometer: A pilot cross-sectional study. Biosci Trends. 2023 Mar 11;17(1):73–7. doi: 10.5582/bst.2022.01476
- Phillips M, Cataneo RN, Condos R, Ring Erickson GA, Greenberg J, La Bombardi V, et al. Volatile biomarkers of pulmonary tuberculosis in the breath. Tuberculosis (Edinb). 2007 Jan;87(1):44–52. doi: 10.1016/j.tube.2006.03.004
- Syhre M, Chambers ST. The scent of Mycobacterium tuberculosis. Tuberculosis (Edinb). 2008 Jul;88(4):317–23. doi: 10.1016/j.tube.2008.01.002
- Phillips M, Basa-Dalay V, Bothamley G, Cataneo RN, Lam PK, Natividad MPR, et al. Breath biomarkers of active pulmonary tuberculosis. Tuberculosis (Edinb). 2010 Mar;90(2):145–51. doi: 10.1016/j.tube.2010.01.003
- Banday KM, Pasikanti KK, Chan ECY, Singla R, Rao KVS, Chauhan VS, et al. Use of urine volatile organic compounds to discriminate tuberculosis patients from healthy subjects. Anal Chem. 2011 Jul 15;83(14):5526–34. doi: 10.1021/ac200265g
- McNerney R, Mallard K, Okolo PI, Turner C. Production of volatile organic compounds by mycobacteria. FEMS Microbiology Letters. 2012 Mar 1;328(2):150–6. doi: 10.1111/j.1574-6968.2011.02493.x
- Mgode GF, Weetjens BJ, Nawrath T, Cox C, Jubitana M, Machang’u RS, et al. Diagnosis of Tuberculosis by Trained African Giant Pouched Rats and Confounding Impact of Pathogens and Microflora of the Respiratory Tract. J Clin Microbiol. 2012 Feb;50(2):274–80. doi: 10.1128/JCM.01199-11
- Crespo E, de Ronde H, Kuijper S, Pol A, Kolk AHJ, Cristescu SM, et al. Potential biomarkers for identification of mycobacterial cultures by proton transfer reaction mass spectrometry analysis. Rapid Commun Mass Spectrom. 2012 Mar 30;26(6):679–85. doi: 10.1002/rcm.6139
- Kolk AHJ, van Berkel JJBN, Claassens MM, Walters E, Kuijper S, Dallinga JW, et al. Breath analysis as a potential diagnostic tool for tuberculosis. Int J Tuberc Lung Dis. 2012 Jun;16(6):777–82. doi: 10.5588/ijtld.11.0576
- Nawrath T, Mgode GF, Weetjens B, Kaufmann SHE, Schulz S. The volatiles of pathogenic and nonpathogenic mycobacteria and related bacteria. Beilstein J Org Chem. 2012 Feb 22;8:290–9. doi: 10.3762/bjoc.8.31
- Phillips M, Basa-Dalay V, Blais J, Bothamley G, Chaturvedi A, Modi KD, et al. Point-of-care breath test for biomarkers of active pulmonary tuberculosis. Tuberculosis (Edinb). 2012 Jul;92(4):314–20. doi: 10.1016/j.tube.2012.04.002
- Scott-Thomas A, Epton M, Chambers S. Validating a breath collection and analysis system for the new tuberculosis breath test. J Breath Res. 2013 Sep;7(3):037108. doi: 10.1088/1752-7155/7/3/037108
- Ellis CK, Stahl RS, Nol P, Waters WR, Palmer MV, Rhyan JC, et al. A Pilot Study Exploring the Use of Breath Analysis to Differentiate Healthy Cattle from Cattle Experimentally Infected with Mycobacterium bovis. PLOS ONE. 2014 Feb 24;9(2):e89280. doi: 10.1371/journal.pone.0089280
- Mellors TR, Rees CA, Wieland-Alter WF, von Reyn CF, Hill JE. The volatile molecule signature of four mycobacteria species. J Breath Res. 2017 Jun 29;11(3):031002. doi: 10.1088/1752-7163/aa6e06
- Beccaria M, Bobak C, Maitshotlo B, Mellors TR, Purcaro G, Franchina FA, et al. Exhaled human breath analysis in active pulmonary tuberculosis diagnostics by comprehensive gas chromatography-mass spectrometry and chemometric techniques. J Breath Res. 2018 Nov 5;13(1):016005. doi: 10.1088/1752-7163/aae80e
- Beccaria M, Mellors TR, Petion JS, Rees CA, Nasir M, Systrom HK, et al. Preliminary investigation of human exhaled breath for tuberculosis diagnosis by multidimensional gas chromatography – Time of flight mass spectrometry and machine learning. J Chromatogr B Analyt Technol Biomed Life Sci. 2018 Feb 1;1074–1075:46–50. doi: 10.1016/j.jchromb.2018.01.004
- Küntzel A, Oertel P, Fischer S, Bergmann A, Trefz P, Schubert J, et al. Comparative analysis of volatile organic compounds for the classification and identification of mycobacterial species. PLOS ONE. 2018 Mar 20;13(3):e0194348. doi: 10.1371/journal.pone.0194348
- Mellors TR, Nasir M, Franchina FA, Smolinska A, Blanchet L, Flynn JL, et al. Identification of Mycobacterium tuberculosis using volatile biomarkers in culture and exhaled breath. J Breath Res. 2018 Oct 30;13(1):016004. doi: 10.1088/1752-7163/aacd18
- Brebu M, Simion VE, Andronie V, Jaimes-Mogollón AL, Beleño-Sáenz K de J, Ionescu F, et al. Putative volatile biomarkers of bovine tuberculosis infection in breath, skin and feces of cattle. Mol Cell Biochem. 2023 Nov;478(11):2473–80. doi: 10.1007/s11010-023-04676-5
- Nol P, Ionescu R, Geremariam Welearegay T, Barasona JA, Vicente J, de Jesus Beleño-Sáenz K, et al. Evaluation of Volatile Organic Compounds Obtained from Breath and Feces to Detect Mycobacterium tuberculosis Complex in Wild Boar (Sus scrofa) in Doñana National Park, Spain. Pathogens. 2020 May 2;9(5):346. doi: 10.3390/pathogens9050346
- Vishinkin R, Busool R, Mansour E, Fish F, Esmail A, Kumar P, et al. Profiles of Volatile Biomarkers Detect Tuberculosis from Skin. Adv Sci (Weinh). 2021 Aug;8(15):e2100235. doi: 10.1002/advs.202100235
- Beccaria M, Franchina FA, Nasir M, Mellors T, Hill JE, Purcaro G. Investigating Bacterial Volatilome for the Classification and Identification of Mycobacterial Species by HS-SPME-GC-MS and Machine Learning. Molecules. 2021 Jul 29;26(15):4600. doi: 10.3390/molecules26154600
- Bobak CA, Kang L, Workman L, Bateman L, Khan MS, Prins M, et al. Breath can discriminate tuberculosis from other lower respiratory illness in children. Sci Rep. 2021 Feb 1;11(1):2704. doi: 10.1038/s41598-021-80970-w
- Makhubela PCK, Rohwer ER, Naudé Y. Detection of tuberculosis-associated compounds from human skin by GCxGC-TOFMS. J Chromatogr B Analyt Technol Biomed Life Sci. 2023 Dec 1;1231:123937. doi: 10.1016/j.jchromb.2023.123937
- Rodríguez-Hernández P, Cardador MJ, Ríos-Reina R, Sánchez-Carvajal JM, Galán-Relaño Á, Jurado-Martos F, et al. Detection of Mycobacterium tuberculosis complex field infections in cattle using fecal volatile organic compound analysis through gas chromatography-ion mobility spectrometry combined with chemometrics. Microbiol Spectr. 2023 Sep 13;11(5):e0174323. doi: 10.1128/spectrum.01743-23
Catch up on the presentations from the Breath Biopsy Conference 2024
