
Liver Breath Analysis (LIBRA®)
Pending Regulatory Approval
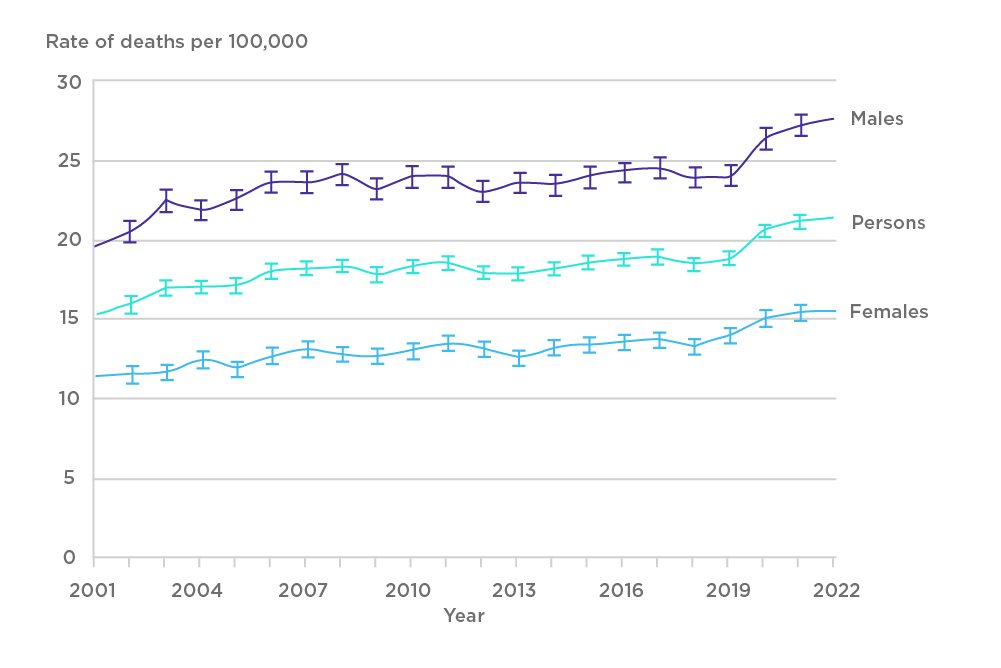
Chronic liver disease (CLD) constitutes a spectrum of disorders ranging from viral hepatitis, alcoholic liver disease (ALD), metabolic dysfunction-associated steatohepatitis (MASH) to autoimmune hepatitis. The progression of these conditions often culminates in liver cirrhosis, a significant cause of morbidity and mortality worldwide[1,2]. In the UK, more than 10,000 deaths annually are attributed to liver disease, which is the equivalent to over 27 deaths per day[3]. Despite extensive testing and improved treatments for hepatitis B and C infections, mortality rates have increased fourfold over the last 50 years, perpetuated by increasing alcohol intake (causing ALD), sedentary lifestyles (causing MASH), and comorbidities (leading ones include obesity, type II diabetes, and atherosclerotic disease).
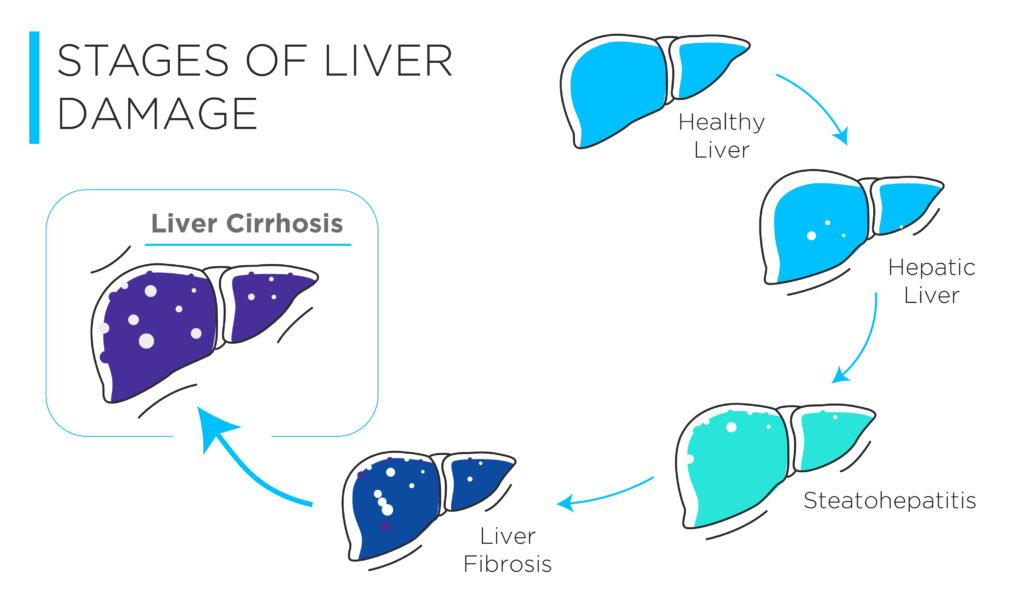
Cirrhosis marks the final stage of progressive fibrosis and is characterised by extensive scarring and formation of regenerative nodules in the liver parenchyma[5]. In the initial stages, cirrhosis is compensated, meaning that liver functions are largely preserved, and the patients often appear asymptomatic, making diagnosis difficult. However, as insults to the liver accumulate, cirrhosis can progress into a decompensated state, often accompanied by the occurrence of complications, such as oesophageal varices, ascites, liver failure, and liver cancer[6].
Decompensated cirrhosis is associated with severe mortality with a median survival of two years, a significant reduction from the 12 years associated with the early, compensated stage[9]. Unfortunately, up to 75% of patients with cirrhosis are only diagnosed at crisis point when they present to accident and emergency (A&E) with life-threatening complications[3]. Treatment for cirrhosis largely depends on its aetiology and stage of cirrhosis. Whilst early interventions – such as lifestyle modifications, antiviral medications for hepatitis, and steroid-based drugs for autoimmune disease – can address the underlying pathophysiology and prevent severe complications, later treatments are limited to managing complications, such as the use of antihypertensives to reduce blood pressure and minimize bleeding risk. This highlights the importance of an early diagnosis[10]. In addition, there is emerging data from clinical trials of novel therapeutics demonstrating promising results in improving fibrosis, which prompts the need for an effective diagnostic test[11].
Diagnosing and clinically intervening compensated cirrhosis is associated with significantly improved prognostic outcomes, when compared to decompensated cirrhosis.
Current Diagnostics
A screening program can increase early cirrhosis detection by 59%, yet detecting asymptomatic cirrhosis in a primary care setting remains challenging[12]. The main factor is the lack of a diagnostic approach that encompasses clinical robustness, cost-effectiveness, and patient adherence. Although liver biopsy is the gold standard in the diagnosis of many liver diseases, it is highly invasive, costly, and carries the risk of infection, making it unsuitable for widespread screening. In addition, it is not feasible for patients to do repeated biopsies to monitor treatment efficacy[13,14]. Although transient elastography is easy to perform, its high capital costs and technical requirements limit its penetration into primary healthcare settings[15,16]. In contrast, blood-based diagnostics are less costly but are ineffective at identifying early stages of fibrosis or assessing disease severity. Their diagnostic performance, measured by sensitivity and specificity, is highly dependent on selecting the appropriate parameters for score calculation. High sensitivity and specificity can only be achieved when using blood biomarkers that are specific to the patient’s etiology. It is noteworthy that most studies have been restricted to hepatitis-C induced cirrhosis, highlighting the limitations of serum biomarkers across diverse patient populations[17,18].
LIBRA® Test
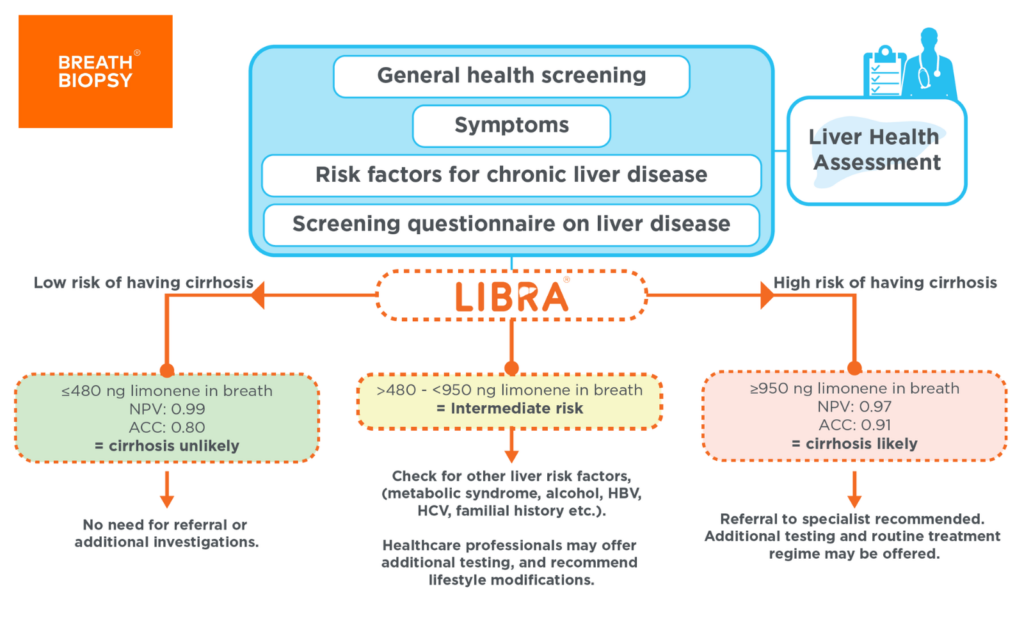
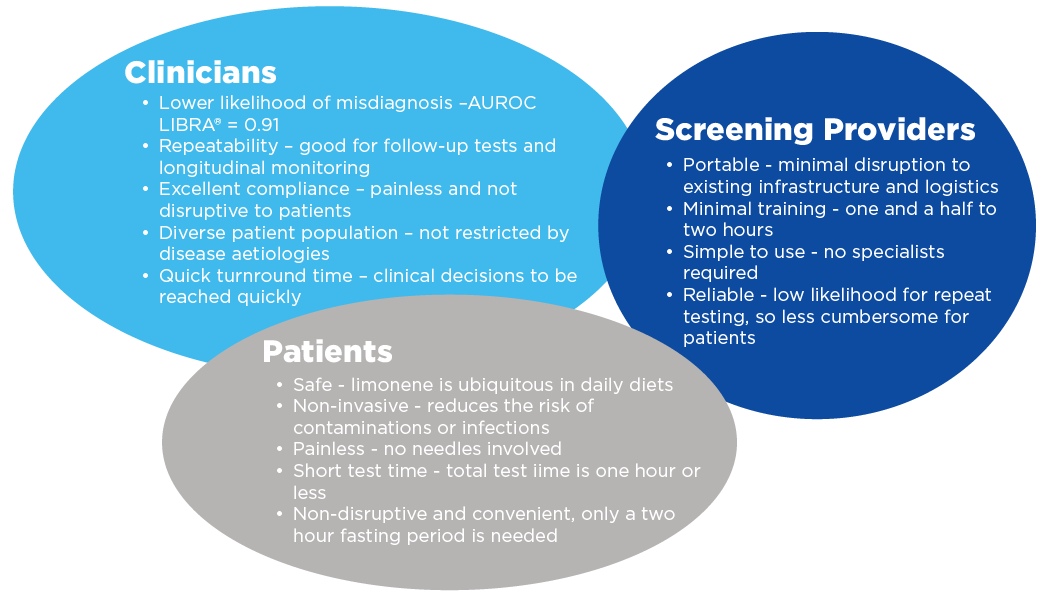
The LIBRA® test has been developed to serve as a non-invasive breath test that enables healthcare professionals to detect liver cirrhosis earlier. Its simplicity, user-friendliness, and lack of requirement for prolonged fasting make it an ideal option for use in primary care settings. Healthcare professionals will be readily able to carry out the test when they identify patients who are at high risk of developing cirrhosis such as obesity, type 2 diabetes, viral hepatitis, and excessive alcohol intake or are symptomatic. The testing process is straightforward: patients ingest a beverage containing limonene (The LIBRA® Beverage) and a breath collection is performed 40 minutes later. The sample is then sent for analysis. The report informs the healthcare professional whether further follow-up tests are needed to confirm cirrhosis diagnosis.

The LIBRA® Beverage
Exhaled breath contains volatile organic compounds (VOCs) that can inform us about our pathological status[19]. Limonene, the active ingredient in the LIBRA® Beverage, is a naturally occurring chemical in citrus fruits. Following ingestion, it is metabolized by CYP2C9/19 enzymes in the liver to a more soluble compound for urine excretion[20]. Patients with cirrhosis typically present with elevated breath limonene levels due to their impaired liver function[21].

The LIBRA® test is our patented innovative solution, enabling healthcare professionals to detect liver cirrhosis earlier, including those with high risk factors (eg: obesity, type 2 diabetes, viral hepatitis and excessive alcohol intake).
Case Study and Sample Test Report


The benefits of LIBRA® make it a viable diagnostic for widespread use in primary care settings.
Next Steps
Our preliminary results have validated the use of limonene as an effective test probe for early cirrhosis detection. In 2025, we will be launching the LIBRA® test. If you are a healthcare professional or healthcare provider who is interested in offering the test, please get in touch.
Further Resources
- FLYER: LIBRA – Targeted probe-based test for non-invasive liver disease detection
- RESOURCE: British Liver Trust: risk of liver disease questionnaire
- PAPER: Exogenous Volatile Organic Compound (EVOC®) Breath Testing Maximizes Classification Performance for Subjects with Cirrhosis and Reveals Signs of Portal Hypertension
- PAPER: Breath Biopsy® to Identify Exhaled Volatile Organic Compounds Biomarkers for Liver Cirrhosis Detection
- PAPER: Breath Biopsy Assessment of Liver Disease Using an Exogenous Volatile Organic Compound—Toward Improved Detection of Liver Impairment
- POSTER: HiSorb TD-GC-MS Innovation: Probing Liver Metabolism with Deuterated EVOCs Unveils Potential Novel Enzymatic Pathways and Biomarkers for Disease Diagnosis
- POSTER: Targeted Breath Biopsy® profiling of induced biomarkers unveils a metabolic adaptation in cirrhosis toward alcohol production
- POSTER: Dynamic Limonene Breath Testing Maximizes Classification Performance for Subjects with Cirrhosis
- POSTER: Pre-clinical exogenous volatile organic compounds (EVOC®) Probes screening and optimization for chronic liver diseases detection
- POSTER: Breath Biopsy® to discover novel exogenous volatile organic compound (EVOC®) biomarkers for chronic liver disease
- RESOURCE: Breath and the Liver 101 Document
References
1. Sepanlou SG, Safiri S, Bisignano C, Ikuta KS, Merat S, Saberifiroozi M, et al. The global, regional, and national burden of cirrhosis by cause in 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. The Lancet Gastroenterology & Hepatology. 2020 Mar 1;5(3):245–66. doi: 10.1016/S2468-1253(19)30349-8
2. PubMed entry [Internet]. [cited 2024 Sep 5]. Available from: http://www.ncbi.nlm.nih.gov/pubmed/31981519
3. Liver disease in numbers – key facts and statistics [Internet]. British Liver Trust. [cited 2024 Sep 5]. Available from: https://britishlivertrust.org.uk/information-and-support/statistics/
4. GOV.UK [Internet]. [cited 2024 Sep 5]. Liver disease profile, April 2024 update. Available from: https://www.gov.uk/government/statistics/liver-disease-profile-april-2024-update/liver-disease-profile-april-2024-update
5. Sharma B, John S. Hepatic Cirrhosis. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 [cited 2024 Sep 5]. Available from: http://www.ncbi.nlm.nih.gov/books/NBK482419/
6. Cirrhosis of the liver [Internet]. British Liver Trust. [cited 2024 Sep 5]. Available from: https://britishlivertrust.org.uk/information-and-support/liver-conditions/cirrhosis/
1. Sepanlou SG, Safiri S, Bisignano C, Ikuta KS, Merat S, Saberifiroozi M, et al. The global, regional, and national burden of cirrhosis by cause in 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. The Lancet Gastroenterology & Hepatology. 2020 Mar 1;5(3):245–66. doi: 10.1016/S2468-1253(19)30349-8
2. PubMed entry [Internet]. [cited 2024 Sep 5]. Available from: http://www.ncbi.nlm.nih.gov/pubmed/31981519
3. Liver disease in numbers – key facts and statistics [Internet]. British Liver Trust. [cited 2024 Sep 5]. Available from: https://britishlivertrust.org.uk/information-and-support/statistics/
4. GOV.UK [Internet]. [cited 2024 Sep 5]. Liver disease profile, April 2024 update. Available from: https://www.gov.uk/government/statistics/liver-disease-profile-april-2024-update/liver-disease-profile-april-2024-update
5. Sharma B, John S. Hepatic Cirrhosis. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 [cited 2024 Sep 5]. Available from: http://www.ncbi.nlm.nih.gov/books/NBK482419/
6. Cirrhosis of the liver [Internet]. British Liver Trust. [cited 2024 Sep 5]. Available from: https://britishlivertrust.org.uk/information-and-support/liver-conditions/cirrhosis/
7. Feng G, Valenti L, Wong VWS, Fouad YM, Yilmaz Y, Kim W, et al. Recompensation in cirrhosis: unravelling the evolving natural history of nonalcoholic fatty liver disease. Nat Rev Gastroenterol Hepatol. 2024 Jan;21(1):46–56. doi: 10.1038/s41575-023-00846-4
8. Healthline [Internet]. 2018 [cited 2024 Sep 5]. Liver Fibrosis. Available from: https://www.healthline.com/health/liver-fibrosis
9. Prognosis | Background information | Cirrhosis | CKS | NICE [Internet]. [cited 2024 Sep 5]. Available from: https://cks.nice.org.uk/topics/cirrhosis/background-information/prognosis/
10. Cirrhosis of the liver [Internet]. British Liver Trust. [cited 2024 Sep 5]. Available from: https://britishlivertrust.org.uk/information-and-support/liver-conditions/cirrhosis/
11. Harrison SA, Bedossa P, Guy CD, Schattenberg JM, Loomba R, Taub R, et al. A Phase 3, Randomized, Controlled Trial of Resmetirom in NASH with Liver Fibrosis. New England Journal of Medicine. 2024 Feb 7;390(6):497–509. doi: 10.1056/NEJMoa2309000
12. Labenz C, Arslanow A, Nguyen-Tat M, Nagel M, Wörns MA, Reichert MC, et al. Structured Early detection of Asymptomatic Liver Cirrhosis: Results of the population-based liver screening program SEAL. Journal of Hepatology. 2022 Sep 1;77(3):695–701. doi: 10.1016/j.jhep.2022.04.009
13. PubMed entry [Internet]. [cited 2024 Sep 5]. Available from: http://www.ncbi.nlm.nih.gov/pubmed/29262175
14. Pandey N, Hoilat GJ, John S. Liver Biopsy. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 [cited 2024 Sep 5]. Available from: http://www.ncbi.nlm.nih.gov/books/NBK470567/
15. Bajre M, Moawad M, Shumbayawonda E, Carolan JE, Hart J, Culver E, et al. LiverMultiScan as an alternative to liver biopsy to monitor autoimmune hepatitis in the National Health Service in England: an economic evaluation. BMJ Open. 2022 Sep 1;12(9):e058999. doi: 10.1136/bmjopen-2021-058999
16. Armstrong MJ, Corbett C, Hodson J, Marwah N, Parker R, Houlihan DD, et al. Operator training requirements and diagnostic accuracy of Fibroscan in routine clinical practice. Postgrad Med J. 2013 Dec;89(1058):685–92. doi: 10.1136/postgradmedj-2012-131640
17. Sharma S, Khalili K, Nguyen GC. Non-invasive diagnosis of advanced fibrosis and cirrhosis. World J Gastroenterol. 2014 Dec 7;20(45):16820–30. doi: 10.3748/wjg.v20.i45.16820
18. Soresi M, Giannitrapani L, Cervello M, Licata A, Montalto G. Non invasive tools for the diagnosis of liver cirrhosis. World J Gastroenterol. 2014 Dec 28;20(48):18131–50. doi: 10.3748/wjg.v20.i48.18131
19. Moura PC, Raposo M, Vassilenko V. Breath volatile organic compounds (VOCs) as biomarkers for the diagnosis of pathological conditions: A review. Biomed J. 2023 Aug;46(4):100623. doi: 10.1016/j.bj.2023.100623
20. PubMed entry [Internet]. [cited 2024 Sep 5]. Available from: http://www.ncbi.nlm.nih.gov/pubmed/11950794
21. Ferrandino G, Orf I, Smith R, Calcagno M, Thind AK, Debiram-Beecham I, et al. Breath Biopsy Assessment of Liver Disease Using an Exogenous Volatile Organic Compound—Toward Improved Detection of Liver Impairment. Clin Transl Gastroenterol. 2020 Sep 16;11(9):e00239. doi: 10.14309/ctg.0000000000000239
