Unveiling Methane’s Intricate Role in Broader Physiological Systems
The appreciation of methane’s biological role is shifting, from being viewed as an end-product of microbiota metabolism to a molecule with implications for endogenous processes.
| Research Area: Methane in the body
Application: Breath analysis Sample medium: Breath Summary:
|
Introduction
Methane is primarily thought to be produced in the body by methanogenic archaea in the gastrointestinal tract of certain individuals, where it may influence digestion. Emerging data has added to this view, both supporting wider physiological impacts of methane, and new sources beyond the gut microbiota. Expanding our understanding of methane’s origins and physiological effects is therefore crucial for exploring its broader health implications. In a recent review by Kerr et al., (1) methane’s origins, physiological effects, and clinical implications in human physiology were explored.
In this case study we will discuss this review and current research findings, to better understand the mechanisms underlying methane production as well as the impact of these mechanisms of gastrointestinal function, immune modulation, and metabolic pathways.
Sources of Methane in Human Breath
Gut Microbiome
The gut microbiome metabolizes dietary and endogenous components in the large intestine, producing volatile compounds, including gases and short-chain fatty acids (SCFAs) such as acetate, propionate, and butyrate. Despite the widespread biological interest surrounding these SCFAs, they are only responsible for <1% of total gas in the gut, with carbon dioxide, methane and hydrogen making up the remaining 99%. Methanogenic archaea, primarily Methanobrevibacter smithii (M. smithii), are the main methane producers in the gut via the methylotrophic pathway (2), with the microbiomes of people classed as high methane producers can have a 1000-fold increase in M. smithii (3).
Once produced, methane diffuses into the bloodstream and is partially (~20-50%) exhaled, enabling hydrogen methane breath tests (HMBTs) to assess gastrointestinal health and diagnose disorders such as intestinal methanogen overgrowth (IMO). A challenge for these breath tests is the reliance on a single measurement set. Recent developments in the field of breath analysis, such as the OMED Health Breath Analyzer, allow for longitudinal measurements of breath methane.
Human Endogenous Processes
Beyond methane production by gut methanogens, emerging data has demonstrated additional endogenous sources that may contribute to measurable methane levels, particularly in settings of biological stress. In particular, studies have demonstrated the extraction of methane from methyl-containing substrates by reactive oxygen species (ROS) via the Fenton reaction, with this process having an overall anti-oxidative effect (4).
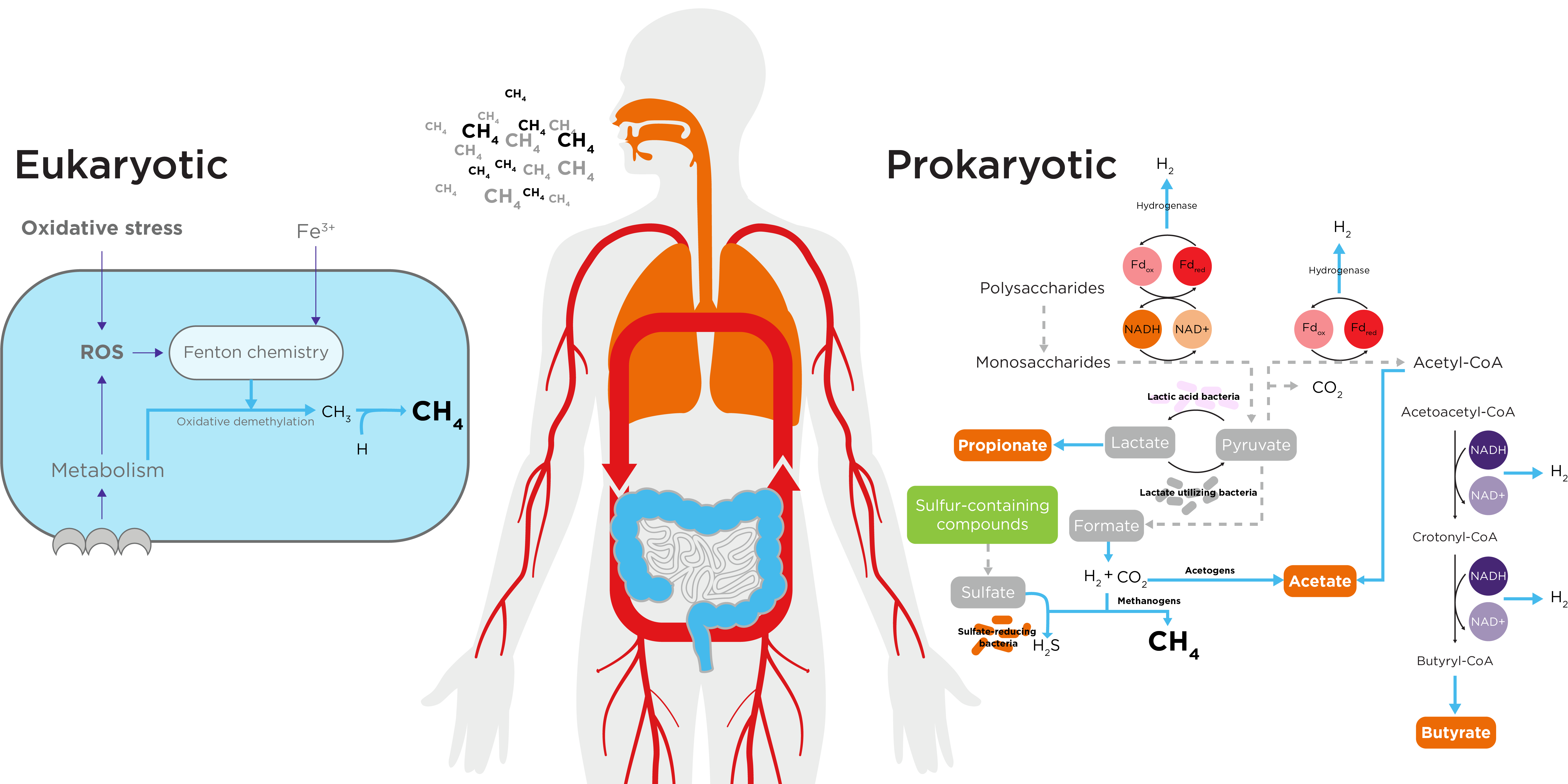
Figure 1. Proposed routes for both exogenous and endogenous methane production.
Demonstrating the relationship between oxidative settings and methane production ex vivo studies have applied oxidative stress to isolated mitochondria, resulting in induced methane production, a process which could be prevented by the application of catalase (5). The potential relevance of these findings to human physiology was observed in a study by Tuboly et al., who demonstrated that the administration of LPS in mice (a model reflecting acute systemic inflammation and commonly used to study sepsis) corresponded with a 2-3-fold increase in methane production (6). This suggests that acute increases in inflammation/oxidative stress, such as in the setting of infection, may provide real-world settings for elevations in non-microbial methane production.
At the Breath Biopsy Conference 2024, Dr. Daniela Polag presented her work on ‘What does breath methane tell us?’. Dr. Polag discusses studies that have demonstrated methane’s interactions throughout the body. You can watch Dr. Polag’s talk, as well as the rest of the presentations from BBCon 2024, here.
Potential Effects of Methane
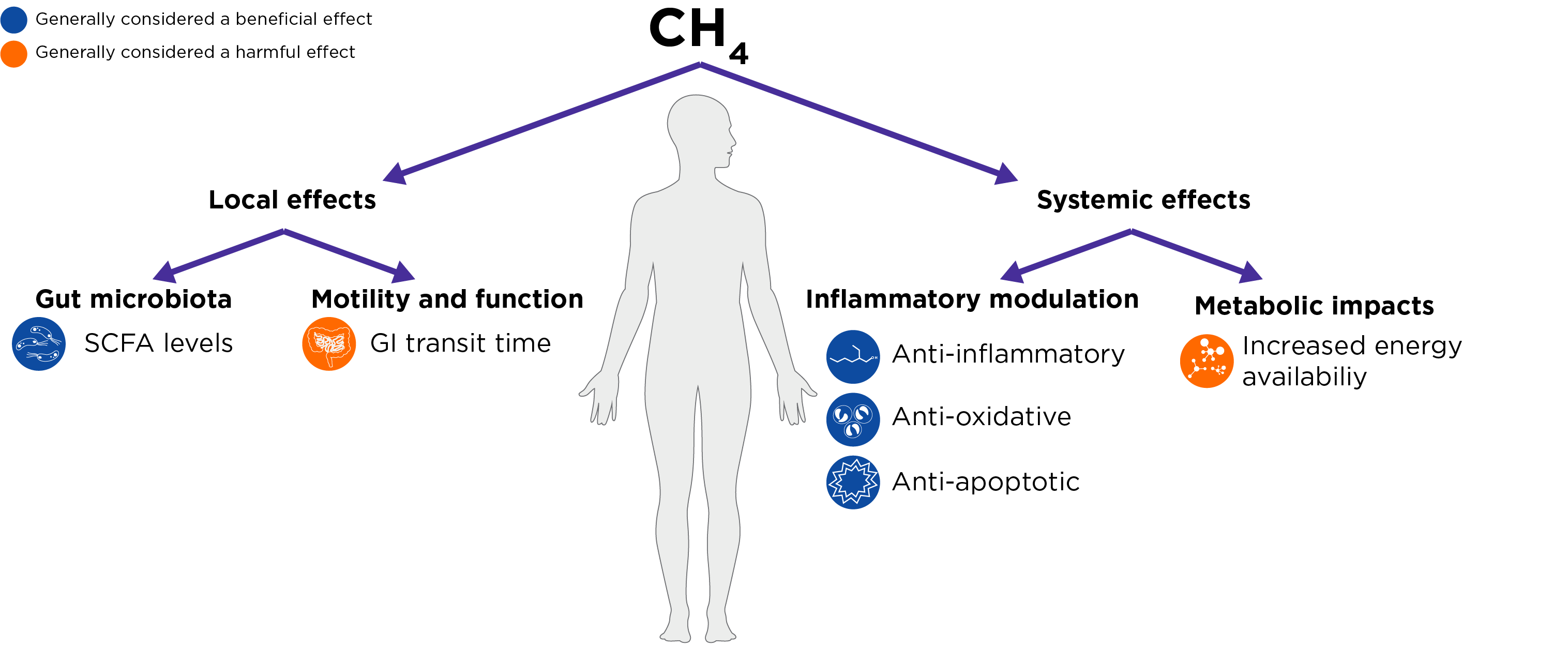
Figure 2. The local and systemic effects proposed for methane within the field. Dark blue icons are generally considered as having a beneficial effect, and orange icons are generally considered as having a harmful effect.
Gastrointestinal Effect
Data from animal studies suggests that methane can have a profound impact on gastrointestinal transit time. Exogenous methane gas applied in ex vivo has been shown to increase intestinal transit times by 59% in dogs and decrease peristaltic velocity in guinea pigs (7). This finding can be translated to observations in human populations, with methane levels correlating strongly with slower intestinal transit times (8). A study by Soares et al., found that total colonic transit time averaged 80.5 hours in methane producers compared to 61.0 hours in non-methane producers (9). Whilst work is ongoing here, it is thought that methane decreases ileal and colon transit speed by raising the amplitude of contraction and slowing peristalsis.
Studies have found that high methane production is associated with a significantly higher microbiome diversity and composition compared to low-methane production (3). High methane producers also show increased levels of formate and acetate in the gut (10). However, results have been conflicting across studies, with some finding methane and SCFA levels positively correlated and some finding a negative correlation. Age could be a factor playing a part in these conflicting results, as age is known to correlate with both increased methane production and decreased SCFA levels. In studies where age is balanced between participant groups, a positive relationship between methane and SCFA production is demonstrated (11).
Systemic Effect
The most common systemic effect attributed to methane is its potential for inflammatory modulation. Studies have associated methane with an anti-inflammatory effect, anti-oxidative effect, and anti-apoptotic effect. These effects have been observed across a wide range of diseases, including ischemia (12), inflammatory disease (13), and others. However, it is worth noting that these studies generally leverage supra-physiological concentrations of methane, and therefore further work is required to demonstrate the physiological relevance of this association.
Studies have found that the gut microbiomes of obese mice have an increased representation of archaea compared to control-weight mice (14). There are two effects of methane that could contribute towards additional weight gain, and therefore create a positive correlation between BMI and methane production. Firstly, slowed gastrointestinal transit time, providing greater time for nutrient absorption across the gastrointestinal tract, and secondly an increased production of SCFAs increasing calorie availability from food. One study also found that methane producers had worse glucose tolerance compared to non-methane producers, and that pharmacologically reducing breath methane (using antibiotics) in obese patients improved glucose tolerance (15).
Conclusion
Methane’s potential roles in human physiology are diverse and complex, spanning from gastrointestinal health to systemic inflammatory and oxidative responses. As research progresses, the potential of methane as both a diagnostic marker and a therapeutic target becomes increasingly evident. The development of handheld, real-time breath methane monitors has enabled large-scale, longitudinal data collection. This opens the door to studies on the role of breath methane in infection, a deeper understanding of correlations with lifestyle factors, and how methane levels change in response to interventions such as antibiotics or treatments. Exploring methane’s origins, mechanisms, and health effects promises new insights for diagnostics and treatments.
At Owlstone Medical we have many tools and services that can incorporate breath analysis into your research, including our handheld hydrogen and methane device, and Breath Biopsy® Collection Station. Our Breath Biopsy VOC Atlas® has currently more than 200 rigorously identified VOCs, including gut microbiome-associated VOCs including SCFAs such as acetate, butyrate, and propionate. You can sign up and gain access to our VOC Atlas here.
References
- Beyond the Gut: Unveiling Methane’s Role in Broader Physiological Systems – [v1] [Internet]. [cited 2024 Nov 29]. Available from: https://www.preprints.org/manuscript/202411.0391/v1
- Nkamga VD, Henrissat B, Drancourt M. Archaea: Essential inhabitants of the human digestive microbiota. Human Microbiome Journal. 2017 Mar 1;3:1–8. doi: 10.1016/j.humic.2016.11.005
- Kumpitsch C, Fischmeister FPS, Mahnert A, Lackner S, Wilding M, Sturm C, et al. Reduced B12 uptake and increased gastrointestinal formate are associated with archaeome-mediated breath methane emission in humans. Microbiome. 2021 Sep 24;9(1):193. doi: 10.1186/s40168-021-01130-w
- Ghyczy M, Torday C, Boros M. Simultaneous generation of methane, carbon dioxide, and carbon monoxide from choline and ascorbic acid: a defensive mechanism against reductive stress? FASEB J. 2003 Jun;17(9):1124–6. doi: 10.1096/fj.02-0918fje
- Ghyczy M, Torday C, Kaszaki J, Szabó A, Czóbel M, Boros M. Hypoxia-induced generation of methane in mitochondria and eukaryotic cells: an alternative approach to methanogenesis. Cell Physiol Biochem. 2008;21(1–3):251–8. doi: 10.1159/000113766
- Tuboly E, Szabó A, Erős G, Mohácsi A, Szabó G, Tengölics R, et al. Determination of endogenous methane formation by photoacoustic spectroscopy. J Breath Res. 2013 Dec;7(4):046004. doi: 10.1088/1752-7155/7/4/046004
- Pimentel M, Lin HC, Enayati P, van den Burg B, Lee HR, Chen JH, et al. Methane, a gas produced by enteric bacteria, slows intestinal transit and augments small intestinal contractile activity. Am J Physiol Gastrointest Liver Physiol. 2006 Jun;290(6):G1089-1095. doi: 10.1152/ajpgi.00574.2004
- Attaluri A, Jackson M, Valestin J, Rao SSC. Methanogenic flora is associated with altered colonic transit but not stool characteristics in constipation without IBS. Am J Gastroenterol. 2010 Jun;105(6):1407–11. doi: 10.1038/ajg.2009.655
- Soares ACF, Lederman HM, Fagundes-Neto U, de Morais MB. Breath methane associated with slow colonic transit time in children with chronic constipation. J Clin Gastroenterol. 2005 Jul;39(6):512–5. doi: 10.1097/01.mcg.0000165665.94777.bd
- Ruaud A, Esquivel-Elizondo S, de la Cuesta-Zuluaga J, Waters JL, Angenent LT, Youngblut ND, et al. Syntrophy via Interspecies H2 Transfer between Christensenella and Methanobrevibacter Underlies Their Global Cooccurrence in the Human Gut. mBio. 2020 Feb 4;11(1):e03235-19. doi: 10.1128/mBio.03235-19
- Fernandes J, Wang A, Su W, Rozenbloom SR, Taibi A, Comelli EM, et al. Age, dietary fiber, breath methane, and fecal short chain fatty acids are interrelated in Archaea-positive humans. J Nutr. 2013 Aug;143(8):1269–75. doi: 10.3945/jn.112.170894
- Liu L, Sun Q, Wang R, Chen Z, Wu J, Xia F, et al. Methane attenuates retinal ischemia/reperfusion injury via anti-oxidative and anti-apoptotic pathways. Brain Res. 2016 Sep 1;1646:327–33. doi: 10.1016/j.brainres.2016.05.037
- Jia Y, Li Z, Feng Y, Cui R, Dong Y, Zhang X, et al. Methane-Rich Saline Ameliorates Sepsis-Induced Acute Kidney Injury through Anti-Inflammation, Antioxidative, and Antiapoptosis Effects by Regulating Endoplasmic Reticulum Stress. Oxid Med Cell Longev. 2018;2018:4756846. doi: 10.1155/2018/4756846
- Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006 Dec 21;444(7122):1027–31. doi: 10.1038/nature05414
- Mathur R, Chua KS, Mamelak M, Morales W, Barlow GM, Thomas R, et al. Metabolic effects of eradicating breath methane using antibiotics in prediabetic subjects with obesity. Obesity (Silver Spring). 2016 Mar;24(3):576–82. doi: 10.1002/oby.21385
Microbiome Content Pack: exclusive content on how breath analysis can be used for gut microbiome research
