Identification of Potential Perihilar Cholangiocarcinoma Biomarkers to Help with Differential Diagnosis
This study evaluated whether volatile organic compounds (VOCs) in bile samples could be emerging diagnostic biomarkers for perihilar cholangiocarcinoma
| Publication information: Xinru Gui, Xin, Zhang, Yiwei Xin, Qi Liu, Yifeng Wang, Yanli Zhang, Yunfei Xu, Zengli Liu, Helgi B. Schioth, Chengxi Sun, Zongli Zhang, Yi Zhang. Identification and validation of volatile organic compounds in bile for differential diagnosis of perihilar cholangiocarcinoma.
Disease Area: Perihilar Cholangiocarcinoma. Sample Medium: Bile. Analysis Approach: GC-IMS. Summary:
|
Introduction – Perihilar Cholangiocarcinoma
Cholangiocarcinoma is a group of malignant tumors originating from epithelial cells in the bile duct and can be split into intrahepatic, perihilar, or distal depending on their anatomical site. Perihilar cholangiocarcinoma (PHCCA) accounts for around 50% of all cholangiocarcinoma (1). The diagnosis of PHCCA is challenging because the symptoms are non-specific, such as weight loss and abdominal pain, which can be found in many other gastrointestinal tract tumors.
Most patients with PHCCA will show signs of hyperbilirubinemia, where there is a build-up of bilirubin in the blood, causing a yellow discoloration of the eyes and skin, known as jaundice (2). Current diagnostic approaches for PHCCA are limited due to the absence of disease-specific symptoms in the early stages. CA19-9 is a serum biomarker for cholangiocarcinoma, however, its low specificity and sensitivity limit its wide application as a biomarker for PHCCA (3).
These issues (non-specific symptoms and biomarkers) lead to a late diagnosis and poor prognosis of PHCCA. Most patients are diagnosed at advanced stages, and the prognosis after conventional treatment is poor, with a 5-year survival rate for 7-20% of patients, with malignant recurrence common (4). There is an urgent need to develop new methods for diagnosing PHCCA.
Volatile organic compounds (VOCs) can be detected in various biological samples, such as serum, urine, bile, and exhaled breath, and there is an increasing interest in VOCs as potential biomarkers for diagnosis and treatment monitoring of disease. For example, VOCs detected in exhaled breath can be used to distinguish lung cancer patients from healthy controls (5), and VOCs detected in urine have been reported in esophageal cancers (6). The collection and analysis of VOCs in bodily samples through techniques such as GC-IMS, provides an emerging non-invasive method for cancer diagnosis.
Bile is secreted by liver cells and flows through the bile duct tree, accumulates in the gallbladder, and plays a crucial role in fat digestion and absorption. When tumorigenesis occurs in the body, it can lead to abnormal up or down-regulation of signal pathways in the body and be reflected in the change of components in bile. Therefore, these changes can indicate the occurrence of disease. A recent study by Gui et al. found different VOCs in bile between PHCCA patients and benign biliary disease (BBD) patients and constructed a novel approach for PHCCA diagnosis.
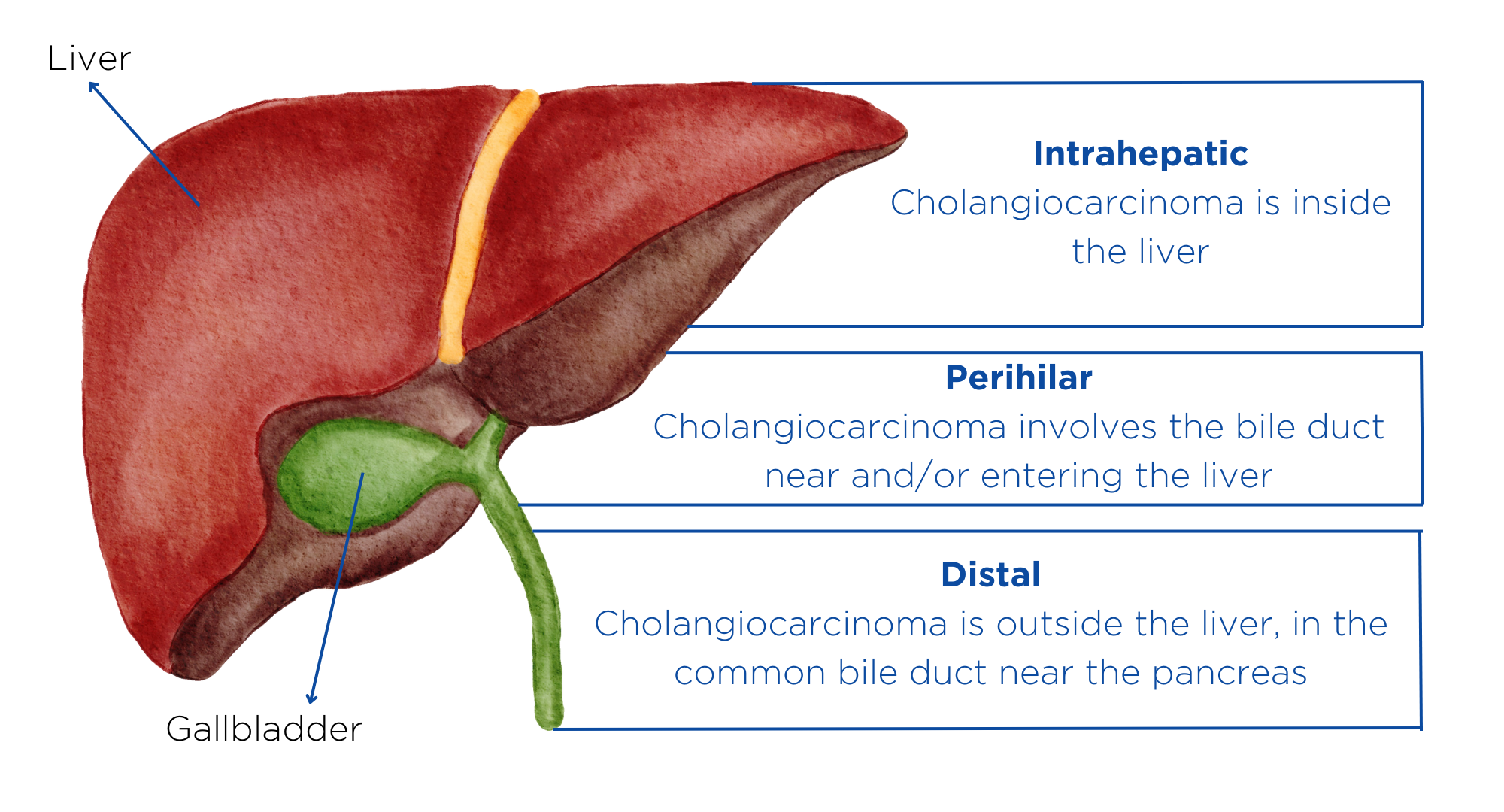
Figure 1. The intrahepatic subtype of cholangiocarcinoma starts in the bile duct inside the liver and typically appears as a mass in the liver, which may affect the organ’s function. The second subtype, called perihilar cholangiocarcinoma, occurs just outside of the liver, where the bile ducts come together and exit the liver. Distal cholangiocarcinoma is the third subtype. It occurs in the portion of the bile duct that is outside of the liver and nearest to the intestine. Adapted from the Mayo Clinic
Methods
Study design and participants
In the training cohort, 73 PHCCA patients and 67 BBD patients were enrolled in this study. Bile samples were collected for all patients and detected using GC-IMS to identify candidate VOCs and construct diagnostic models. The test cohort was comprised of 29 patients with PHCCA and 31 patients with BBD. These participants were used to evaluate the diagnostic models. Therefore, 102 patients were recruited in the disease group (PHCCA) and 98 BBD patients, including 55 cases with bile duct stones, and 43 cases with cholangitis.
Sample preparation
Bile samples were collected from patients receiving endoscopic retrograde cholangiopancreatography (ERCP) or percutaneous transhepatic cholangiodrainage (PTCD). The bile samples were centrifuged at 3000g, 4°C, 10 minutes, and stored at -80°C.
Analysis of VOCs in bile
GC-IMS was used for VOC analysis in bile samples. Headspace gas from the air surrounding the bile samples was extracted for further analysis. Each analysis was conducted in triplicate.
Results
Identification of VOC signals detected in bile of patients with PHCCA and BBD
A total of 43 VOC signal peaks were preliminarily selected in all training bile samples. Among them, 32 signal peaks were statistically significant with p < 0.05. There were 19 defined VOC substances, and 5 unknown substances analyzed and used for further analysis.
A big difference was seen between PHCCA and BBD bile samples. However, this difference was not seen among different stages of PHCCA, or between bile duct stones and cholangitis. The suggested VOCs have potential as biomarkers for PHCCA diagnosis.
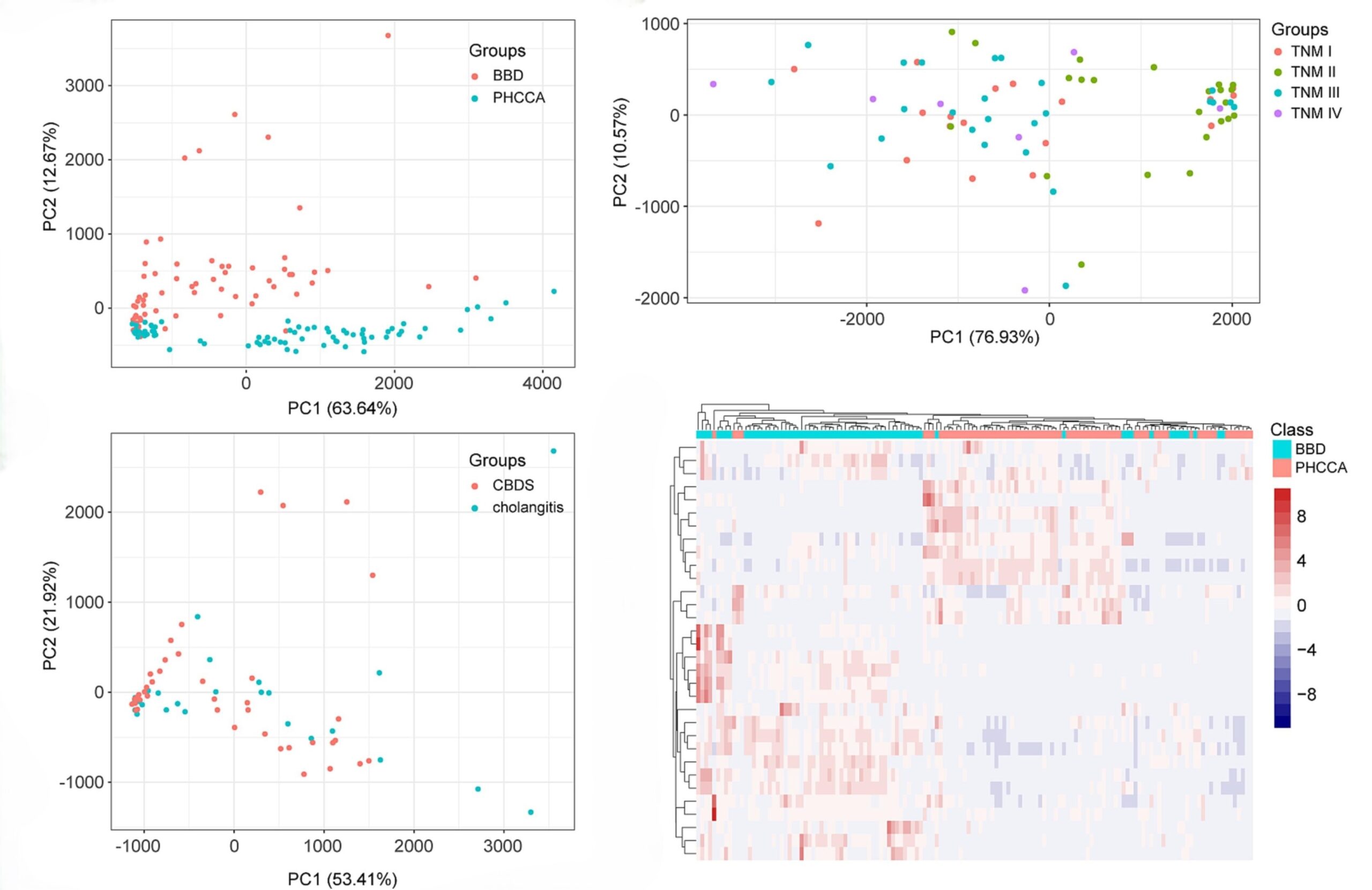
Figure 2. Graphs highlighting the principal component analysis (PCA) which shows an obvious difference between PHCCA and BBD and that unsupervised hierarchical clustering analysis also demonstrates a legible segregation in PHCCA and BBD. Figures from Gui et al.
Quantitative analysis of biliary VOCs and their comparison between PHCCA and BBD
Compared with BBD, four VOCs were significantly up-regulated in PHCCA, including 2-ethyl-1-hexanol, propyl isovalerate, cyclohexanone, and acetophenone. Eight VOCs were down-regulated, including (E)-2-hexenal, (E)-2-octenal, (E)-2-pentenal, 2-methoxyfuran, diethyl malonate, hexanal, methyl acetate, and (E)-hept-2-enal.
Diagnostic performance of bile VOCs for PHCCA in training and test cohorts
ROC curve analysis found that the 12 VOCs described above could distinguish PHCCA and BBD.
VOC biomarkers in bile and pathological parameters
To investigate whether the changes of VOCs in bile are related to tumorigenesis and progression, this study collected detailed pathological data of cancer patients in the test set and analyzed the relationship between the 12 identified VOCs and pathological indicators. Acetophenone showed a significant difference in tumor size. Hexenal and 2-methoxyfuran presented significant differences in tumor differentiation.
Discussion
The early diagnosis of PHCCA is critical for successful treatment and better disease prognosis. The research discussed in this case study used a two-phase study to identify and validate a VOC panel for PHCCA diagnosis. Based on the VOCs identified in this study, machine learning algorithms provided a 93.1% sensitivity and 100% specificity in distinguishing PHCCA from BBD.
In the discovery phase, 32 signal peaks from 12 VOC substances showed statistical differences between PHCCA patients and BBD patients. Analysis suggested bile VOCs had potential as biomarkers for PHCCA diagnosis.
A total of 12 VOCs were identified in PHCCA patients compared with BBD patients, including one type of alcohol, two types of ketones, three types of esters, five types of aldehydes, and 2-methoxyfuran. Some of the VOCs identified in this study have also been reported in other cancers. The VOCs 2-ethyl-1-hexanol and acetophenone were reported to distinguish different lung cancer cell lines – for example, acetophenone could differentiate non-small cell lung cancer from small cell lung cancer, and 2-ethyl-1-hexanol could differentiate adenocarcinoma and squamous cell carcinoma (7).
The different VOC trends between PHCCA and BBD might imply abnormal biochemical reactions when patients suffer from cancer or inflammation. A series of redox reactions within cells involves a generation of aldehydes, alcohols, ketones, and esters. Alcohols can generate aldehydes or ketones after the oxidation-reduction reaction, while ketones cannot be further oxidized. One alcohol, 2-ethyl-1-hexanol, and two ketones were up-regulated in the cancer group in this study, indicating that more redox reactions due to inflammation are occurring in PHCCA patients (1).
The compounds found in this study, including alcohols, aldehydes, and ketones are all examples of VOCs that have been clinically validated to be found in exhaled breath, and can be found in our developing internal reference library – the Breath Biopsy VOC Atlas®. The Atlas is a searchable database of chemically identified VOCs with quantitative data from previous human studies, including cohorts of healthy and disease subjects. The Atlas allows us to build an understanding of the biological processes the VOCs found in breath are involved with. Therefore, data from the Atlas can be used to accelerate biomarker development. You can gain access to the Atlas to support your research here.
The collection and analysis of VOCs in breath present an alternative and complementary technique to VOCs in bile. At Owlstone Medical, we have the expertise to implement breath VOC analysis, providing both breath sampling collection hardware, analysis, and biological interpretation of VOCs in the context of what is known about their mechanistic origin based on internal data and the broader literature.
This includes state-of-the-art high-resolution accurate mass TD-GC-MS through our Breath Biopsy OMNI® platform. Our OMNI service is rigorously tested at all stages to provide robust results that support volatile analysis for biomarker identification, validation, translation, and clinical implementation.
References
- Gui X, Zhang X, Xin Y, Liu Q, Wang Y, Zhang Y, et al. Identification and validation of volatile organic compounds in bile for differential diagnosis of perihilar cholangiocarcinoma. Clin Chim Acta. 2023 Feb 15;541:117235. doi: 10.1016/j.cca.2023.117235
- Watson RL. Hyperbilirubinemia. Critical Care Nursing Clinics of North America. 2009 Mar 1;21(1):97–120. doi: 10.1016/j.ccell.2008.11.001
- Chang JC, Kundranda M. Novel Diagnostic and Predictive Biomarkers in Pancreatic Adenocarcinoma. Int J Mol Sci. 2017 Mar 20;18(3):667. doi: 10.3390/ijms18030667
- Banales JM, Marin JJG, Lamarca A, Rodrigues PM, Khan SA, Roberts LR, et al. Cholangiocarcinoma 2020: the next horizon in mechanisms and management. Nat Rev Gastroenterol Hepatol. 2020 Sep;17(9):557–88. doi: 10.1038/s41575-020-0310-z
- Chen X, Muhammad KG, Madeeha C, Fu W, Xu L, Hu Y, et al. Calculated indices of volatile organic compounds (VOCs) in exhalation for lung cancer screening and early detection. Lung Cancer. 2021 Apr;154:197–205. doi: 10.1016/j.lungcan.2021.02.006
- Huang J, Kumar S, Abbassi-Ghadi N, Spaněl P, Smith D, Hanna GB. Selected ion flow tube mass spectrometry analysis of volatile metabolites in urine headspace for the profiling of gastro-esophageal cancer. Anal Chem. 2013 Mar 19;85(6):3409–16. doi: 10.1021/ac4000656
- Barash O, Peled N, Tisch U, Bunn PA, Hirsch FR, Haick H. Classification of lung cancer histology by gold nanoparticle sensors. Nanomedicine. 2012 Jul;8(5):580–9. doi: 10.1016/j.nano.2011.10.001
