Designing Studies to Standardize Breath Biomarker Discovery
Published on: 20 Sep 2021
The compounds present in exhaled breath have great potential as clinical biomarkers with wide ranging applications for early detection, precision medicine and drug development. Yet, despite numerous research projects and promising pilot studies, relatively few breath tests are currently available for clinical use. Pick up any review of breath biomarker research, and you’re likely to see clear reasons why1,2,3,4,5. Largely it comes down to limited reproducibility of results due to differing study designs. The number of sampling protocols, chemical analysis and statistical approaches that have been applied to finding breath biomarkers is almost as great as the number of potential biomarkers themselves, so it’s no surprise that comparing results between them has become a key challenge.
While the connection between breath biomarkers and environmental factors can be a significant advantage in breath sampling, it also poses a challenge for consistent results. It’s easy for volatile compounds to make their way into a breath sample without being relevant for breath analysis. Once collected, there’s no easy way to distinguish biorelevant compounds in a breath sample from contaminants that can confound the detection of potentially valuable biomarkers. As such, it’s clear that exploring and establishing standards in breath research and data reporting is a critical step in the continued efforts towards discovery and validation of clinically-relevant breath biomarkers.
Method standardization was a key discussion topic at the 2021 Breath Biopsy Conference. Recordings of this event are now available to watch on demand:
Standardizing breath data across the community – Oh my BoB!
Breath or Blank (BoB) studies, use a relatively simple study design intended to provide a standardized scientific approach to breath research that can be used in conjunction with any breath sampling and quantitative analysis methodology. Irrespective of approach, BoB studies enable comparable reporting regarding the number of detectable biorelevant VOCs on breath, as well as intra- and inter-subject variability.
|
In BoB studies, we define a compound on breath as being one where the area under the curve in a breath sample is greater than three standard deviations above the average abundance in blank samples. |
The hallmark of a BoB study is the inclusion of a proportionally large number (typically 1:1) of representative blank samples alongside a selection of breath samples, typically involving repeat sampling from a small number of healthy volunteers. These blanks allow determination of all undesired sources of VOCs including from the collection and analysis devices themselves and the ambient environment during breath sampling. This comparison of breath vs. blanks allows VOCs originating on breath to be determined with high confidence and distinguished from compounds that are not clinically relevant.
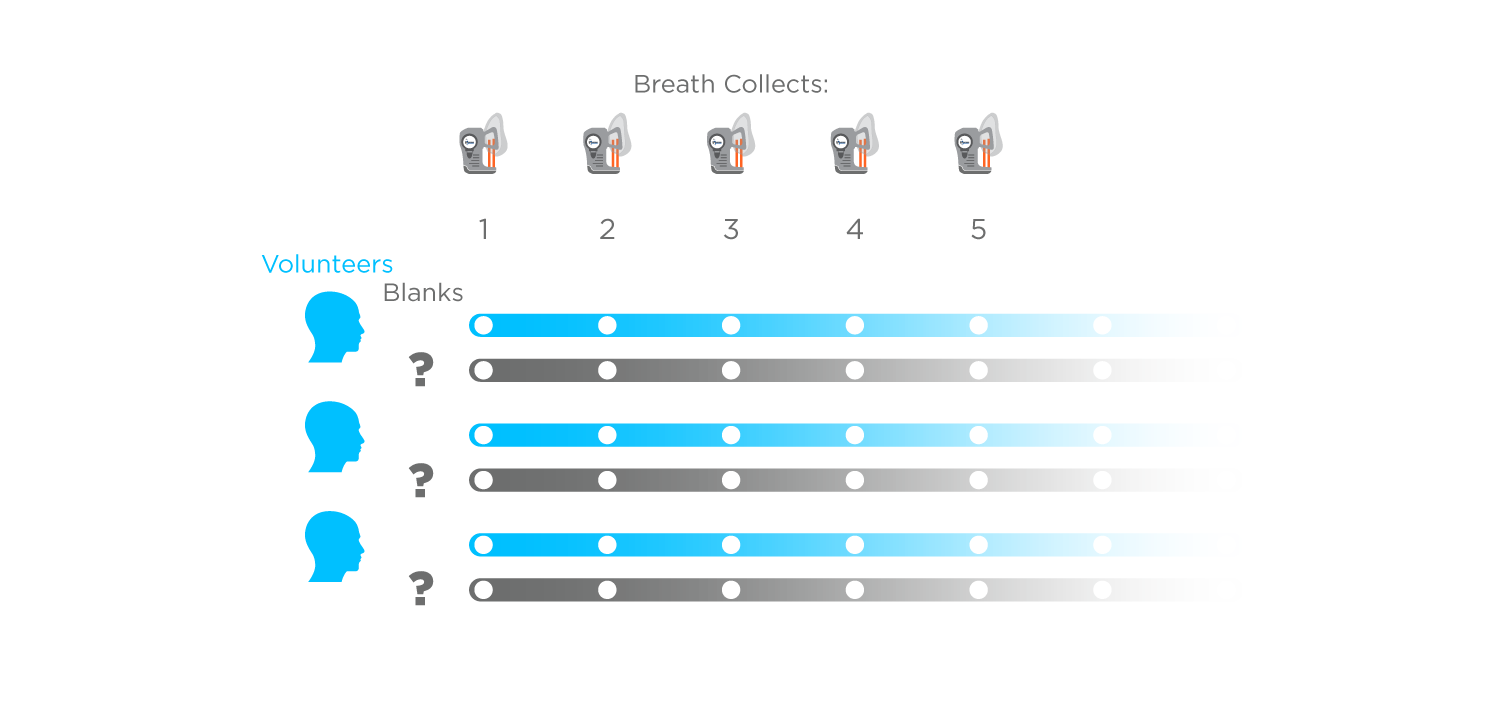
Figure 1: A general design for a Breath or Blank (BoB) study showing multiple samples collected from healthy volunteers and an equivalent number of blank sample collections. Blanks can reflect a range of technical considerations but should represent all possible sources of contamination of a breath sample.
Sampling a relatively uniform population makes it easier to understand the impacts of ambient variation and factors influenced by your collection and analysis methods. Repeat sampling of the same individuals, in a short period of time, represents the best-case scenario, with minimal biological variability. As such, BoB studies can be used to quantify reproducibility and sample-to-sample variation. Most importantly, including many blanks allows biorelevant on-breath compounds to be separated from those that are simply found in breath samples but that bear no relation to patient biology.
BoB to the top
We strongly support the more widespread use and reporting of BoB studies in the breath research field and we believe they offer significant benefits in helping to identify the most appropriate biomarker discovery methods for the full range of research questions currently being explored in the field. Not only do they provide results that can be used to compare diverse methodologies on a level playing field, but they can be used to measure iterative improvements, helping to assess the impacts of individual method changes.
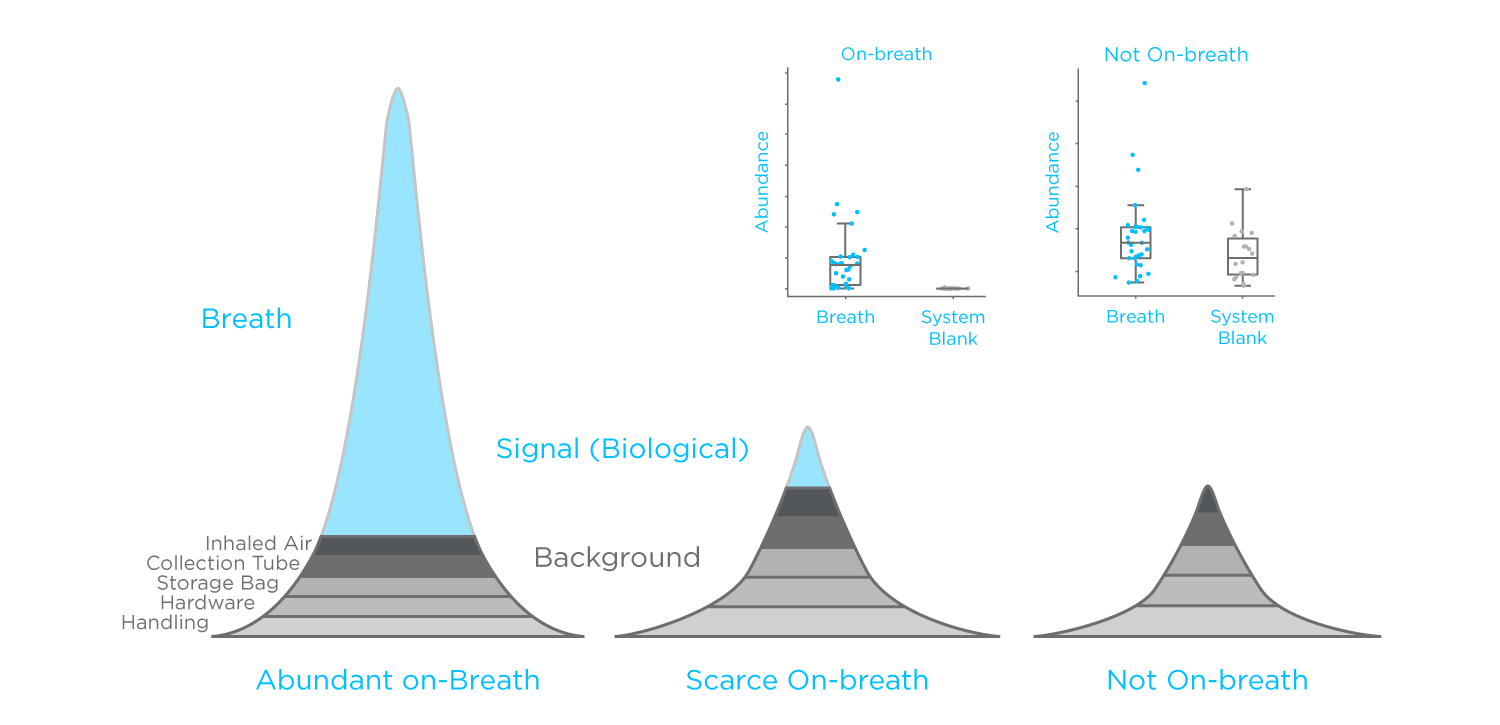
Figure 2: A representation of how different VOC sources can contribute to the peaks on a GC-MS chromatogram of a breath sample. Insert: boxplots representing a generalized on-breath compound vs. a compound not on breath.
The ability to share comparable results in this way would be a significant advance for driving progress and collaboration across the breath research community. Until now, studies have typically reported on the total number of detected compounds without any differentiation in how representative of biological signals. We anticipate that this development will greatly enhance the discovery of relevant biomarkers and will lead to progress in validating and progressing them towards clinical application.
Contact Us about Standardizing Breath Research
References
- Lawal, O., et al., Exhaled breath analysis: a review of ‘breath-taking’ methods for off-line analysis. Metabolomics, 2017. 13(10): p. 110. DOI: 10.1007/s11306-017-1241-8
- Majchrzak, T., et al., Sample preparation and recent trends in volatolomics for diagnosing gastrointestinal diseases. TrAC Trends in Analytical Chemistry, 2018. 108: p. 38-49. DOI: 10.1016/j.trac.2018.08.020
- Van Malderen, K., et al., Volatomics in inflammatory bowel disease and irritable bowel syndrome. EBioMedicine, 2020. 54: p. 102725. DOI: 10.1016/j.ebiom.2020.102725
- Azim, A., et al., Exhaled volatile organic compounds in adult asthma: a systematic review. Eur Respir J, 2019. 54(3). DOI: 10.1183/13993003.00056-2019
- De Vincentis, A., et al., Exhaled breath analysis in hepatology: State-of-the-art and perspectives. World J Gastroenterol, 2019. 25(30): p. 4043-4050. DOI: 10.3748/wjg.v25.i30.4043
