Demystifying VOCs: What You Need to Know About Volatile Organic Compounds
Published on: 18 Jul 2023
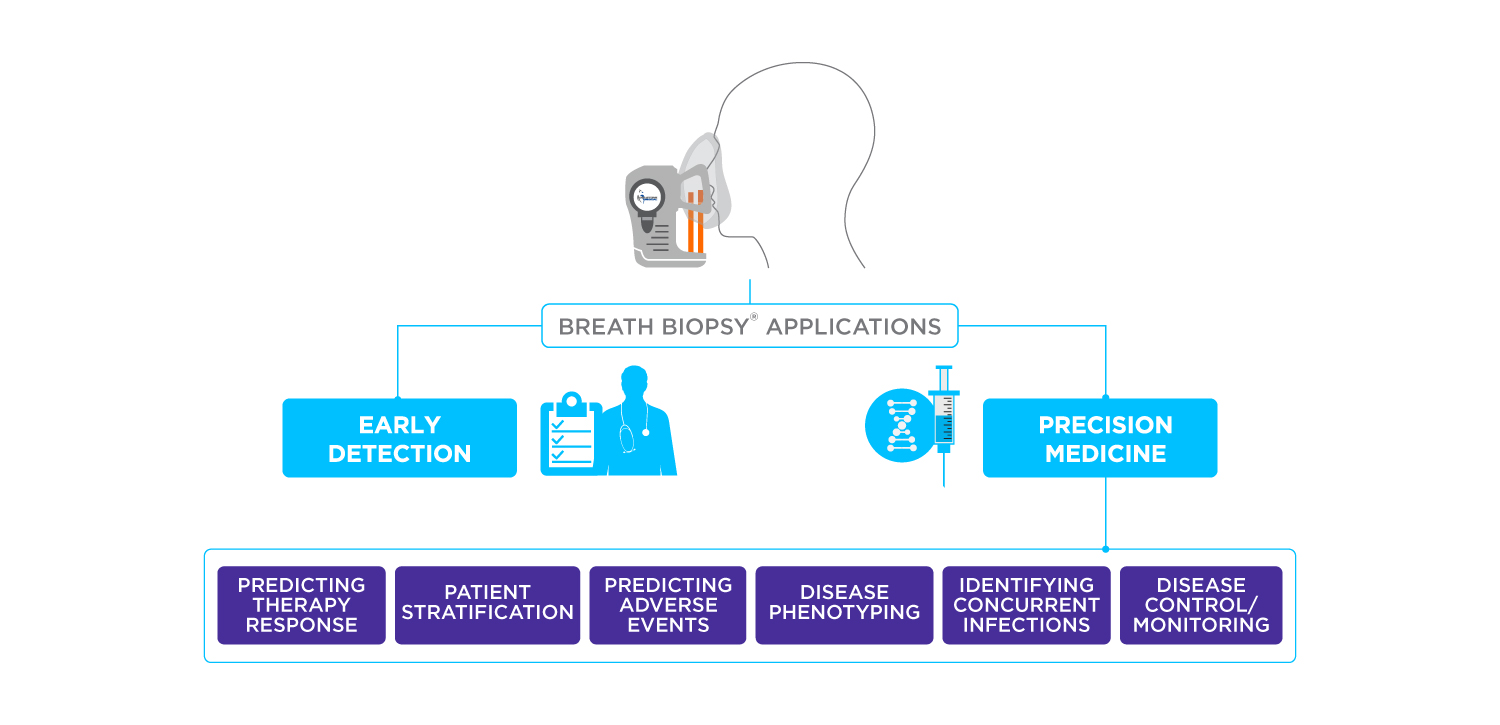
The measurement of volatile organic compounds (VOCs) produced by metabolic activity in the body is a powerful approach for precision medicine and early detection across a wide variety of disease areas. But what is the definition of a VOC?
VOCs refer to a vast group of organic chemicals that readily evaporate at room temperature. These compounds can be found in a wide range of products, including paints, solvents, cleaning agents, and personal care items. In fact, VOCs within the body have natural and human-made origins, including from the body’s own metabolic processes (endogenous VOCs) or from external sources such as diet, the microbiome, prescription drugs, and environmental exposure (exogenous VOCs). VOCs can contribute to air pollution and environmental concerns (1).
These volatile compounds are categorized based on their molecular structure or functional groups, for example, chlorinated, oxygenated, nitrogenous, aromatic hydrocarbons, alcohols, ethers, esters, aldehydes, alkanes, alkenes, ketones, and sulfur-containing compounds (2).
While VOCs have often been associated with industry and environmental impacts, their unique properties make them valuable for medical applications, with the added bonus that their propensity to evaporate enables them to be sampled quickly and non-invasively from exhaled breath. They exhibit a range of physical properties that contribute to their volatility and potential for evaporation:
• VOCs generally have relatively high vapor pressures at room temperature, allowing them to readily transition from a liquid or solid phase to a gaseous phase. The vapor pressure of a VOC depends on factors such as temperature, molecular weight, and intermolecular forces (3).
• The boiling point of a VOC is the temperature at which it changes from a liquid to a gas at atmospheric pressure. VOCs typically exhibit lower boiling points compared to other compounds, rendering them highly volatile. Boiling points of VOCs can span a wide range, from very low temperatures (e.g., -40°C) to higher temperatures (e.g., 200°C or higher) (3).
• Henry’s Law constant represents the ratio of the concentration of a VOC in the gas phase to its concentration in the liquid phase (usually water) at a given temperature. Henry’s Law constant is important for understanding the volatilization of VOCs from liquids and their potential for atmospheric release (4).
• VOCs can exhibit varying solubilities in different substances, such as water or organic solvents. VOCs largely have limited solubility in water, and the solubility of a VOC affects its behavior in aqueous environments (2).
• The molecular weight of VOCs can vary significantly, ranging from very low (e.g., less than 50 g/mol) to high (e.g., several hundred g/mol). Generally, lower molecular weight VOCs tend to be more volatile, while higher molecular weight VOCs have lower volatility (5).
The composition of VOCs in exhaled breath can provide valuable insights into an individual’s metabolic state and health condition, acting as breath biomarkers. By analyzing the VOC profile in breath samples, researchers can potentially diagnose and monitor various diseases.
Breath analysis holds promise in diagnosing and monitoring various states of health and disease in the body. Conditions for which breath analysis can be utilized include respiratory diseases, such as asthma and chronic obstructive pulmonary disease (COPD), as well as detecting various types of cancer and gastrointestinal disorders (6,7).
If you are interested in learning more about the medical applications of VOCs as Breath Biomarkers you can find out more in our on-demand webinar, or if you have any questions please get in touch.
References:
- United States Environmental Protection Agency (EPA). Volatile Organic Compounds’ Impact on Indoor Air Quality. https://www.epa.gov/indoor-air-quality-iaq/volatile-organic-compounds-impact-indoor-air-quality
- Ioanna Katsikantami, Manolis N. Tzatzarakis, Volatile Organic Compounds, Reference Module in Biomedical Sciences, Elsevier, 2022, ISBN 9780128012383, DOI: 10.1016/b978-0-12-824315-2.00095-6.
- Lide, D. R. (Ed.). (2008). CRC Handbook of Chemistry and Physics. CRC Press.
- Schwarzenbach, R. P., Gschwend, P. M., & Imboden, D. M. (2003). Environmental Organic Chemistry (2nd ed.). Wiley-Interscience.
- National Research Council. (2010). Acute Exposure Guideline Levels for Selected Airborne Chemicals: Volume 9. National Academies Press.
- Ibrahim W, Carr L, Cordell R, Wilde MJ, Salman D, Monks PS, Thomas P, Brightling CE, Siddiqui S, Greening NJ. Breathomics for the clinician: the use of volatile organic compounds in respiratory diseases. Thorax. 2021 May;76(5):514-521. doi: 10.1136/thoraxjnl-2020-215667.
- Haick H, Broza YY, Mochalski P, Ruzsanyi V, Amann A. Assessment, origin, and implementation of breath volatile cancer markers. Chem Soc Rev. 2014 Mar 7;43(5):1423-49. DOI: 10.1039/c3cs60329f.
Catch up on the presentations from the Breath Biopsy Conference 2024