Analyzing the changes in breath VOC biomarkers in response to food
Internal case study: Analyzing Metabolic Responses to a Standardized Meal Challenge using the Breath Biopsy® OMNI® Platform
Published on: 2 Jul 2024
Internal case study: Analyzing Metabolic Responses to a Standardized Meal Challenge using the Breath Biopsy® OMNI® Platform
Introduction
Ingestion of food causes dynamic and complex changes to metabolism, and depending on the composition of the food, can contribute to the development of cardiovascular, metabolic, and liver diseases (1–5). Metabolic pathways in the body can produce volatile organic compounds (VOCs), a group of compounds that contain at least one carbon atom and are gaseous at room temperature and standard pressure conditions. VOCs that are generated by endogenous human metabolism can cross most biological membranes away from their point of origin, and be emitted from bodily fluids including breath, urine, and feces (6–8). This means that the VOC composition of exhaled breath could serve as a platform to investigate the metabolic responses to food, and facilitate a deeper understanding of the intricate connection between human metabolism and different physiological states in a non-invasive manner (Figure 1).
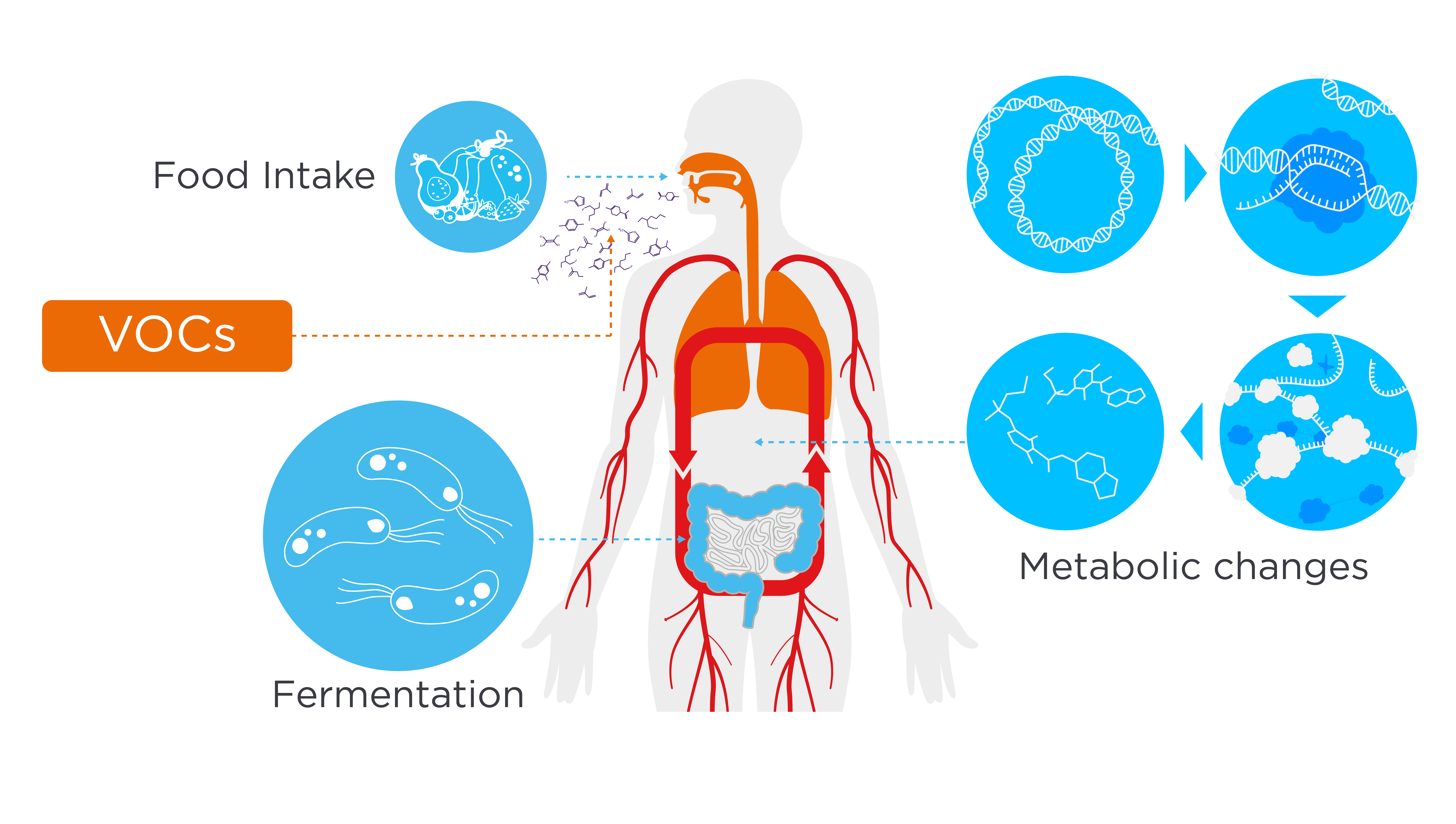
Figure 1 – Metabolism in the body can lead to the production of VOCs detectable in exhaled breath, meaning that breath VOC analysis could provide a novel approach for metabolic research.
To this end, we conducted an internal study to assess the impact of dietary intake on VOCs in exhaled breath, using our OMNI® breath collection and analysis platform. We aimed to identify metabolically relevant VOCs to non-invasively study diet-induced metabolic changes.
Methods
For this study, 20 healthy volunteers (non-smokers without chronic illness) were recruited, and breath samples were collected while the volunteers were in a fasted state (8+ hours without food or liquids other than water) and after consuming a liquid meal to induce a fed-state metabolic transition. The meal contained a balanced 400 kcal meal composed of 19g of fat, 34g of carbohydrate (4g sugars), 20g of protein, and 6g of fiber. Two samples were taken before the meal (fasted 1 and fasted 2) and post-meal (fed 1 at 20 minutes after the meal and fed 2 at 1 hour after the meal). An equipment blank air sample was collected each sampling day for each volunteer to control for interferent VOCs from the background air that could contaminate the samples. The breath and equipment blank samples were compared to identify compounds present on-breath. Matched fed and fasted samples were then compared to identify on-breath VOCs associated with the metabolic-state transition. Thermal desorption gas chromatography-mass spectrometry (TD-GC-MS) was used to analyze the collected breath samples and equipment blanks. VOC chemical identities were identified using our internal High-Resolution Accurate Mass (HRAM) library, and statistical analyses were performed to distinguish breath compounds from ambient air, and the main groups (fasted sample 1/sample 2 vs. fed sample 1/sample 2) were compared using repeated measures ANOVA and Wilcoxon tests. A VOC was defined as on-breath if it exceeds 3 standard deviations above the mean of blanks for that VOC and surpasses this threshold in 50% of samples. Alternatively, a feature is considered on-breath if the p-value is less than 0.05 when a t-test is applied between blank and breath samples, or if the ROC-AUC is greater than 0.8 between breath and blank samples.
Results
A total of 217 VOCs were identified as on-breath, 54 passed by paired t-test, 7 had 50% > mean + 3SD and paired t-testing, 92 had all three. Several microbiome-related and exposure VOCs (deriving from food or flavoring) changed from fasting to refeeding (Figure 2). The short-chain fatty acids (SCFAs) butyric acid, propionic acid, and acetic acid are produced by the gut microbiome and showed an upward trend following refeeding. Other microbiome-related VOCs 1-propanol and 2,3-butanedione also increased, while indole and 3-methylindole, products of microbial fermentation of tryptophan, decreased or remained unchanged after food intake. VOCs linked to exposure, commonly found in food or used as flavoring agents, such as valencene, limonene, and terpenes such as alpha-pinene and beta-pinene, also significantly increased 20 minutes after food consumption and stabilized at the 1-hour timepoint. Additionally, dimethyl disulfide, p-cymene, and 2,3-butanedione are notable microbiome-related compounds that change from fasting to refeeding.
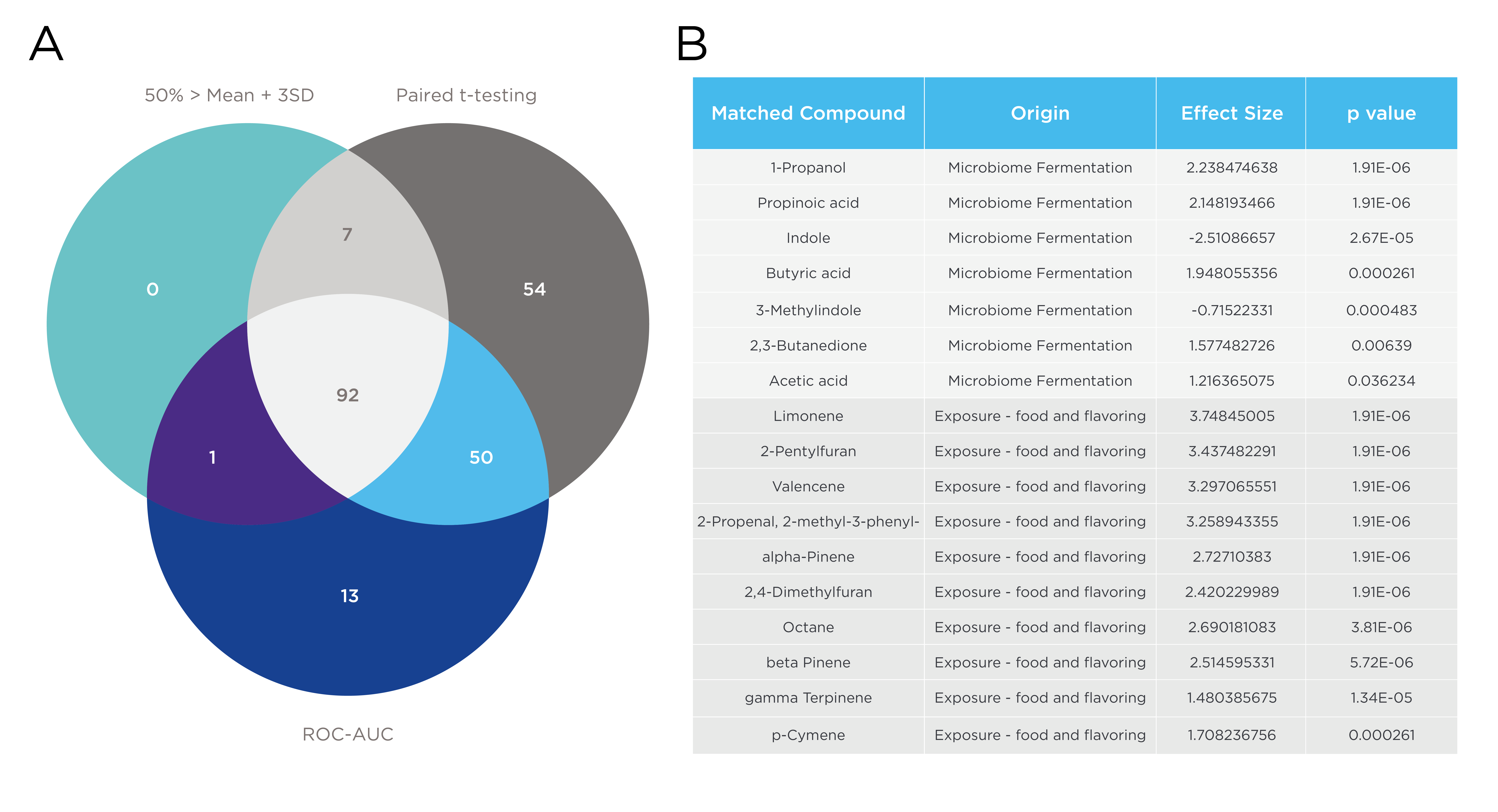
Figure 2 – A: the number of on-breath VOCs by each criterion, B: highlights of the on-breath VOCs with the most significant changes 20 minutes after meal intake.
Dr. Matteo Tardelli recently presented this work at the Breath Biopsy Conference 2024. You can watch his talk on-demand, as well as the other presentations from the conference here.
Discussion
Butyric and propionic acids are produced by the fermentation of dietary fibers by certain gut bacteria, such as members of the Firmicutes phylum (e.g., Clostridia species). These acids play a crucial role in maintaining gut health and have anti-inflammatory properties. Indole and 3-methylindole are products of the bacterial breakdown of the amino acid tryptophan and have multiple roles, including signaling within the gut and influencing host physiology. Various gut bacteria, including Escherichia coli, can produce indole. Indole also has multiple roles, including signaling within the gut which can subsequently influence host physiology.
An interesting finding is that the upregulation of SCFAs only 20 minutes after refeeding was not observed in previous literature using a similar protocol and SIFT-MS approach (9,10). However, another study found that the SCFA producer Faecalibacterium showed differing fasting responses between healthy individuals and those with metabolic syndrome but exhibited consistent growth upon refeeding in both groups (11). This suggests that SCFA profiles can change during fasting (12) and may respond to peristalsis stimuli and colon contractions following a liquid meal, contributing to the observed changes. Additionally, another study observed a negative correlation between exhaled indole and blood glucose when breath samples were collected for six hours after type 1 and type 2 diabetes patients ingested a standardized meal (13), consistent with our current data.
Conclusions
The OMNI service delivers comprehensive and robust identification and analysis of volatile compounds in exhaled breath, encompassing expertise in study design, optimized sample collection, high-confidence identification of compounds against reference libraries, analysis, and biological interpretation of breath VOC data. OMNI is rigorously tested at all stages to provide robust results that support volatile analysis for biomarker identification, validation, and translation.
This study underscores the promising utility of the Breath Biopsy OMNI Platform in advancing our understanding of metabolic responses to dietary stimuli. Notably, the platform demonstrated its ability to identify established nutrition and microbiome-associated metabolites such as SCFAs documented in existing literature, while also revealing novel candidate breath VOC biomarkers associated with the metabolic response to food (including the response of the microbiome). As large volumes of breath can be collected in rapid succession at any time, VOCs associated with metabolism can be investigated at multiple time points after supplementation for longitudinal monitoring.
References
- Zeevi D, Korem T, Zmora N, Israeli D, Rothschild D, Weinberger A, et al. Personalized Nutrition by Prediction of Glycemic Responses. Cell. 2015 Nov 19;163(5):1079–94. doi: 10.1016/j.cell.2015.11.001
- Gallwitz B. Implications of Postprandial Glucose and Weight Control in People With Type 2 Diabetes. Diabetes Care. 2009 Nov;32(Suppl 2):S322–5. doi: 10.2337/dc09-S331
- Bansal S, Buring JE, Rifai N, Mora S, Sacks FM, Ridker PM. Fasting Compared With Nonfasting Triglycerides and Risk of Cardiovascular Events in Women. JAMA. 2007 Jul 18;298(3):309–16. doi: 10.1001/jama.298.3.309
- Ning F, Tuomilehto J, Pyörälä K, Onat A, Söderberg S, Qiao Q. Cardiovascular Disease Mortality in Europeans in Relation to Fasting and 2-h Plasma Glucose Levels Within a Normoglycemic Range. Diabetes Care. 2010;33(10). doi: 10.2337/dc09-2328
- Nishida T, Tsuji S, Tsujii M, Arimitsu S, Haruna Y, Imano E, et al. Oral Glucose Tolerance Test Predicts Prognosis of Patients with Liver Cirrhosis. Official journal of the American College of Gastroenterology | ACG. 2006 Jan;101(1):70. doi: 10.1111/j.1572-0241.2005.00307.x.
- Amann A, Costello B de L, Miekisch W, Schubert J, Buszewski B, Pleil J, et al. The human volatilome: volatile organic compounds (VOCs) in exhaled breath, skin emanations, urine, feces and saliva. J Breath Res. 2014 Jun;8(3):034001. doi: 10.1088/1752-7155/8/3/034001
- Drabińska N, Flynn C, Ratcliffe N, Belluomo I, Myridakis A, Gould O, et al. A literature survey of all volatiles from healthy human breath and bodily fluids: the human volatilome. J Breath Res. 2021 Apr 21;15(3). doi: 10.1088/1752-7163/abf1d0
- Bax C, Lotesoriere BJ, Sironi S, Capelli L. Review and Comparison of Cancer Biomarker Trends in Urine as a Basis for New Diagnostic Pathways. Cancers. 2019 Sep;11(9):1244. doi: 10.3390/cancers11091244
- Rodriguez J, Neyrinck AM, Zhang Z, Seethaler B, Nazare JA, Robles Sánchez C, et al. Metabolite profiling reveals the interaction of chitin-glucan with the gut microbiota. Gut Microbes. 2020 Nov 9;12(1):1810530. doi: 10.1080/19490976.2020.1810530
- Neyrinck AM, Rodriguez J, Zhang Z, Seethaler B, Mailleux F, Vercammen J, et al. Noninvasive monitoring of fibre fermentation in healthy volunteers by analyzing breath volatile metabolites: lessons from the FiberTAG intervention study. Gut Microbes. 2021 Jan 1;13(1):1862028. doi: 10.1080/19490976.2020.1862028
- Maifeld A, Bartolomaeus H, Löber U, Avery EG, Steckhan N, Markó L, et al. Fasting alters the gut microbiome reducing blood pressure and body weight in metabolic syndrome patients. Nat Commun. 2021 Mar 30;12(1):1970. doi: 10.1038/s41467-021-22097-0
- Ozkul C, Yalinay M, Karakan T. Structural changes in gut microbiome after Ramadan fasting: a pilot study. Benef Microbes. 2020 May 11;11(3):227–33. doi: 10.3920/BM2019.0039
- Maurer F, Wolf A, Fink T, Rittershofer B, Heim N, Volk T, et al. Wash-out of ambient air contaminations for breath measurements. J Breath Res. 2014 Jun;8(2):027107. doi: 10.1088/1752-7155/8/2/027107
Catch up on the presentations from the Breath Biopsy Conference 2024
