How the Gut Microbiome Influences Skin Health: Exploring the Gut-Skin Axis
Published on: 1 Oct 2024
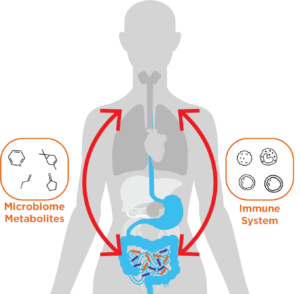
The Gut-skin Axis
It is well-known that diet affects skin health, however, the relationship between the gut and the skin is much more complex than just what we eat. Communication between the gut and the skin is facilitated by a complex series of bidirectional pathways that together form the gut-skin axis. The beneficial microbes that form the gut and skin microbiomes are key components of this axis, with dysregulation of the gut microbiome implicated in a range of different skin conditions. But how does communication through the gut-skin axis actually occur?
As they both act as barriers between the body and the environment, the gut and the skin share similar structures and functions, allowing for crosstalk between them. The main mediators of gut-skin axis communication are the immune system and microbial metabolites. Gut microbiome metabolism produces at least 30 hormone-like compounds, including short-chain fatty acids (SCFAs), cortisol, and neurotransmitters (1). These compounds can travel through the blood to exert their effects on the skin. For instance, gut-secreted propionic acid is toxic to the common skin bacteria staphylococcus aureus (1). The immune system is the key mediator of communication in the gut-skin axis and is heavily modulated by the microbiome. As 70-80% of immune cells in the body are present in the gut, the gut microbiome is particularly important for this modulation (2). It interacts with many aspects of the innate and adaptive immune systems to regulate immune homeostasis (3).
It is well established that dysregulation of the gut microbiota can negatively affect skin health, with gut microbiome dysbiosis able to disrupt immune homeostasis (4). Immune imbalances can cause both local and systemic inflammation and have implicated the gut microbiome in some inflammatory skin conditions (5). Below we discuss key examples of skin diseases are linked to the gut microbiome, focusing on the role of the gut-skin axis in these conditions.
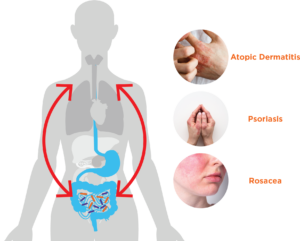
Skin Conditions and the Gut Microbiome
Atopic dermatitis
Atopic dermatitis is the most common inflammatory skin disease, affecting 7% of adults and 15% of children (6). This condition causes skin to become itchy, dry, and cracked, and has been linked to the dysbiosis of the gut microbiome. The modulation of the immune system by microbes is thought to contribute to the pathogenesis of atopic dermatitis (7). Higher levels of Clostridium and Escherichia bacteria were found in the gut of infants with atopic dermatitis (8), and these bacteria are thought to contribute to an inflammatory state (6). Other studies have revealed a decrease in the abundance of SCFAs synthesized by the gut microbiome may increase intestinal permeability and contribute to atopic dermatitis by further triggering inflammatory responses (9).
Psoriasis
Psoriasis is an inflammatory disease that forms silvery-white or grey scales of flaking skin. Chronic inflammation is a characteristic of psoriasis, with levels of inflammatory cytokines increased in skin lesions and blood plasma (10). Alterations to the gut microbiome can contribute to this inflammation through its modulation of the immune system. The decreased presence of Bacteroides in the gut microbiomes of psoriasis patients has been observed (7). These beneficial bacteria are involved in the anti-inflammatory pathway, and therefore a decrease in their presence could induce a pro-inflammatory response. Biomarkers of damaged intestinal barriers have also been observed in psoriasis patients, this also potentially inducing an inflammatory response. Damage to the intestinal barrier is also a characteristic of inflammatory bowel disease (IBD), an inflammatory gut condition that can result in chronic diarrhea and stomach pain. Interestingly, research shows that between 7-11 % of IBD sufferers also have psoriasis (11). This association is thought to be both genetic and immune related, with common inflammatory pathways observed between the diseases.
Rosacea
Rosacea is a long-term inflammatory skin condition characterized by reddened skin of the face. Gastrointestinal symptoms such as bloating, abdominal pain, and altered gut transit time have also been observed in rosacea patients, with the treatment of altered gut transit time in one patient resulting in a complete remission of rosacea (12). Abnormal gut transit time is also a risk factor for small bacterial intestinal overgrowth (SIBO). The link between rosacea and SIBO was investigated by Parodi et al. using a combination of hydrogen and methane breath testing, dermatological analysis, and GI symptom reporting (13). Hydrogen and methane breath tests can accurately reflect the state of the gut microbiome and can indicate SIBO through non-invasive sampling. The results of this study demonstrated a significantly higher presence of SIBO in rosacea patients than controls, with the treatment of SIBO improving the rosacea lesions observed. This indicates a mechanistic link between gut dysbiosis in the small intestine and the pathogenesis of rosacea. These results are explored further in our SIBO and rosacea case study.
Investigating the Gut Microbiome Using Breath Analysis
The key role of the gut-skin axis in some skin diseases indicates potential for the treatment of skin conditions through therapeutics affecting the gut. To determine the efficacy and mechanisms of action of these treatments, dermatological analysis of skin conditions should be correlated with their effects on the microbiome. Breath analysis can provide a non-invasive tool for studying the real-time metabolic activity of the gut microbiome, allowing for the analysis of changing gut conditions in response to therapeutic interventions.
Many volatile organic compounds (VOCs) detectable in the breath are metabolic products of the gut microbiome that may be involved in systemic actions that result in skin conditions. These include SCFAs, indoles, and phenols, which all travel to the lungs via the blood before being exchanged into the breath. These VOCs can be captured from exhaled breath and identified and quantified by high-accuracy gas chromatography-mass spectrometry (GC-MS) using our Breath Biopsy OMNI platform. Furthermore, our Breath Biopsy VOC Atlas®, is a comprehensive catalog of compounds already identified and characterized as being “on-breath” (reliably distinguished from background contaminants), with many linked to the gut microbiome. Combining this database with the OMNI platform our allows for a streamlined study design to accelerate breath biomarker discovery.
Breath Biopsy technology is non-invasive and well tolerated by patients, allowing for frequent sampling in the ongoing monitoring of patients. This allows for increased data collection in clinical trials in comparison to alternative sampling techniques used to study the gut microbiome, such as stool sampling. Additionally, stool samples do not represent the real-time metabolic changes to the microbiome, as can be observed through breath analysis.
We have also developed our OMED Health Breath Analyzer which is suitable for research. This device accurately measures levels of hydrogen and methane and is designed for easy, non-invasive use by patients. These gases are clinically validated biomarkers of gut microbiome activity, and can be used to monitor states of dysbiosis, as demonstrated in the Parodi et al. study investigating SIBO. Monitoring levels of hydrogen and methane exhaled on the breath can evaluate treatment interventions, correlating changes to the gut microbiome with skin symptoms and lifestyle factors.
If you are interested in incorporating breath analysis into your microbiome research, please get in touch.
References
- Chilicka K, Dzieńdziora-Urbińska I, Szyguła R, Asanova B, Nowicka D. Microbiome and Probiotics in Acne Vulgaris—A Narrative Review. Life. 2022 Mar 15;12(3):422. doi: 10.3390/life12030422
- Wiertsema SP, van Bergenhenegouwen J, Garssen J, Knippels LMJ. The Interplay between the Gut Microbiome and the Immune System in the Context of Infectious Diseases throughout Life and the Role of Nutrition in Optimizing Treatment Strategies. Nutrients. 2021 Mar 9;13(3):886. doi: 10.3390/nu13030886
- Zheng D, Liwinski T, Elinav E. Interaction between microbiota and immunity in health and disease. Cell Res. 2020 Jun;30(6):492–506. doi: 10.1038/s41422-020-0332-7
- Wu HJ, Wu E. The role of gut microbiota in immune homeostasis and autoimmunity. Gut Microbes. 2012 Jan 1;3(1):4–14. doi: 10.4161/gmic.19320
- Thye AYK, Bah YR, Law JWF, Tan LTH, He YW, Wong SH, et al. Gut–Skin Axis: Unravelling the Connection between the Gut Microbiome and Psoriasis. Biomedicines. 2022 May;10(5):1037. doi: 10.3390/biomedicines10051037
- De Pessemier B, Grine L, Debaere M, Maes A, Paetzold B, Callewaert C. Gut–Skin Axis: Current Knowledge of the Interrelationship between Microbial Dysbiosis and Skin Conditions. Microorganisms. 2021 Feb 11;9(2):353. doi: 10.3390/microorganisms9020353
- Mahmud MdR, Akter S, Tamanna SK, Mazumder L, Esti IZ, Banerjee S, et al. Impact of gut microbiome on skin health: gut-skin axis observed through the lenses of therapeutics and skin diseases. Gut Microbes. 14(1):2096995. doi: 10.1080/19490976.2022.2096995
- Penders J, Thijs C, van den Brandt PA, Kummeling I, Snijders B, Stelma F, et al. Gut microbiota composition and development of atopic manifestations in infancy: the KOALA Birth Cohort Study. Gut. 2007 May;56(5):661–7. doi: 10.1136/gut.2006.100164
- Xiao X, Hu X, Yao J, Cao W, Zou Z, Wang L, et al. The role of short-chain fatty acids in inflammatory skin diseases. Front Microbiol. 2023 Feb 2;13:1083432. doi: 10.3389/fmicb.2022.1083432
- Systemic Inflammation in Psoriasis: A Guide for Dermatology Care Providers [Internet]. [cited 2024 Aug 6]. Available from: https://practicaldermatology.com/topics/psoriasis/systemic-inflammation-in-psoriasis-a-guide-for-dermatology-care-providers/20439/
- Huang BL, Chandra S, Shih DQ. Skin Manifestations of Inflammatory Bowel Disease. Front Physiol. 2012 Feb 6;3:13. doi: 10.3389/fphys.2012.00013
- Kendall SN. Remission of rosacea induced by reduction of gut transit time. Clin Exp Dermatol. 2004 May;29(3):297–9. doi: 10.1111/j.1365-2230.2004.01461.x
- Parodi A, Paolino S, Greco A, Drago F, Mansi C, Rebora A, et al. Small intestinal bacterial overgrowth in rosacea: clinical effectiveness of its eradication. Clin Gastroenterol Hepatol Off Clin Pract J Am Gastroenterol Assoc. 2008 Jul;6(7):759–64. doi: 10.1016/j.cgh.2008.02.054
Breath Analysis Toolbox: A comprehensive guide on how breath analysis can be used to aid GI microbiome research