The impact of gut dysbiosis on Liver Cirrhosis, Pancreatitis, Parkinson’s, and Cystic Fibrosis
Published on: 20 Feb 2025
Introduction
The human body is home to a large and diverse community of microbes, 95% of which are found in the gut, comprising the gut microbiome (1,2), and are thought to play an important role in health and the development of disease. In particular, the imbalance of the gut microbiome composition, or dysbiosis, is well characterized to be associated with diseases such as irritable bowel syndrome (IBS) and inflammatory bowel disease (IBD) (3). However, the gut microbiome is also thought to be involved in disease processes that affect systems outside of the gastrointestinal tract. This includes metabolic dysfunction-associated fatty liver disease (MAFLD) (4), metabolic dysfunction-associated steatohepatitis (MASH) (5), cardiometabolic diseases (6), and cancer (7,8). If the upper gastrointestinal tract contains an abnormal microbial community (usually one that includes bacteria native to the colon), or if it contains an excessive number of bacteria, a condition known as small intestinal bacterial overgrowth (SIBO) can develop. SIBO symptoms include bloating, diarrhea, and nutrient malabsorption (9). The following blog will explore the link between SIBO and other seemingly unrelated diseases.
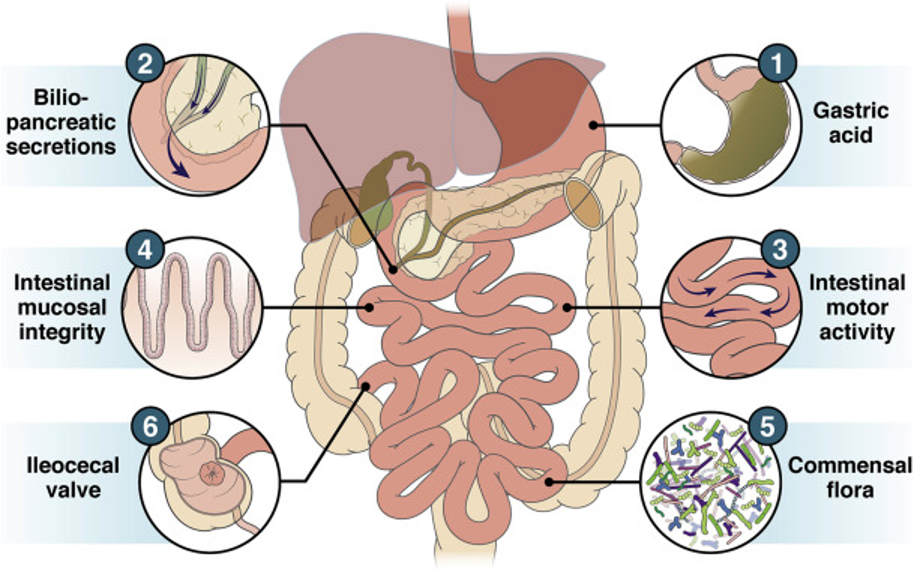
Figure 1: Gastrointestinal tract by Bushyhead and Quigley, 2022 (10).
We previously covered an important study that suggested a mechanistic link between gut dysbiosis of the small intestine (SIBO) to the pathogenesis of rosacea. Rosacea patients had a significantly higher SIBO prevalence than controls and treating their SIBO improved the lesions observed – with the majority of patients maintaining this result for at least nine months. The reasons for this connection were unknown, but it is thought that there is an intricate and dynamic connection between the gut bacteria, immunological regulation, and many organ systems in the body. Consistent with this, the prevalence of SIBO has been associated with other diseases, suggesting that this is an important dysbiosis condition that could be related to the development of disease, or could be a consequence of disease states in the body. SIBO is usually diagnosed through levels of hydrogen and methane in the breath, and through our patient-centric brand OMED Health, we supply the NHS with diagnostic breath testing kits. We have also recently launched a portable hydrogen-methane breath analyzer device, which is suitable for research use for longitudinal tracking of breath composition. In this blog, we explore incidences of SIBO in diseases and discuss how the pathogenesis of the conditions could be related to SIBO.
The connection between SIBO and diseases
Pancreatitis
Pancreatitis involves inflammation of the pancreas, a gland located behind the stomach that has an important role in digestion and regulation of sugar levels in the blood. In chronic pancreatitis, the pancreas becomes inflamed over a long period of time which scars the gland, resulting in reduced exocrine and endocrine activity. The gut microbiota usually has a symbiotic relationship with the human host, however, upon progression of the disease to a more severe form, infections of the pancreas with a high fatality rate are thought to originate from translocated gut microbiota (10,11). Conversely, dysfunction of the exocrine function of the pancreas is known to be a key factor in regulating the composition of the gut microbiota (12). Interestingly, many studies link chronic pancreatitis to SIBO, with a recent meta-analysis reporting that SIBO was more likely to be present in those with pancreatitis than controls (38.6%, 95% CI 25.5%–53.5%) (13). The reasons for this are not clear, however, it is reported that chronic pancreatitis can cause intestinal motility issues including stasis, which is a known risk factor for developing SIBO (13,14).
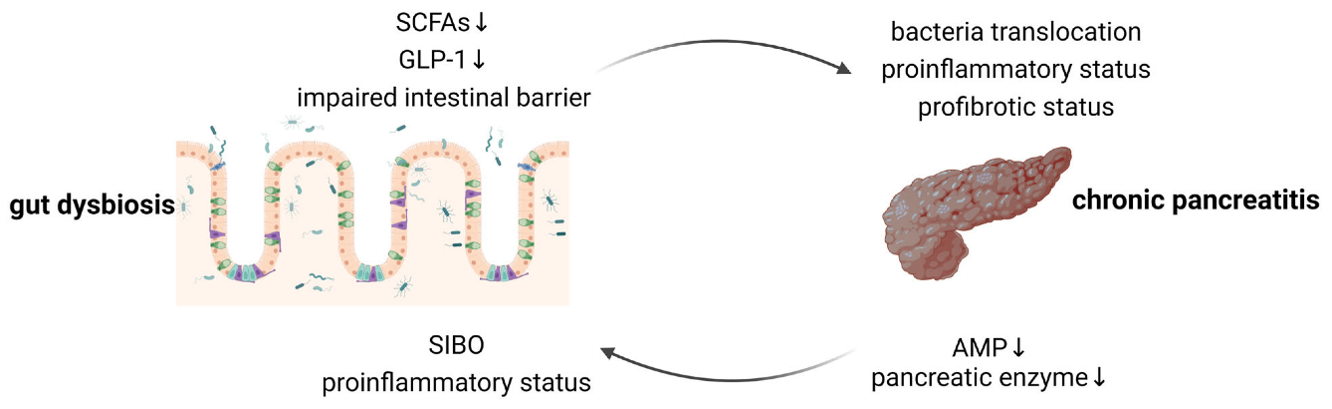
Figure 2: Bidirectional gut–pancreas interactions by Pan et al, 2024 (16).
Cystic Fibrosis
Cystic Fibrosis (CF) is a genetic disease caused by a CFTR (transmembrane conductance regulator) gene mutation. Due to this mutated gene, CF patients have impaired fluid transport across the epithelial cell membranes, leading to thick and viscous mucus buildup at the cell surface, creating an environment prone to colonization and infection by opportunistic pathogens. This primarily affects the lungs, but also impacts other organs that rely on mucus production, such as the gastrointestinal tract. CF is associated with significant alterations to the gut microbiome, and CF patients are more at risk of developing SIBO (30 – 50% prevalence) due to various factors, including sticky mucus blocking pancreatic ducts, altered immunological environment of the small intestine, slower intestinal motility, frequent antibiotic, and proton pump inhibitor use (15–18). As discussed in the previous section, a higher prevalence of SIBO is associated with disrupted pancreatic function. When CF patients with pancreatic insufficiency are excluded (50%), the prevalence of SIBO is nearer to 30%, however, this is still higher than the prevalence reported in the literature for healthy controls (0 – 20%) (16,19).
Liver Cirrhosis
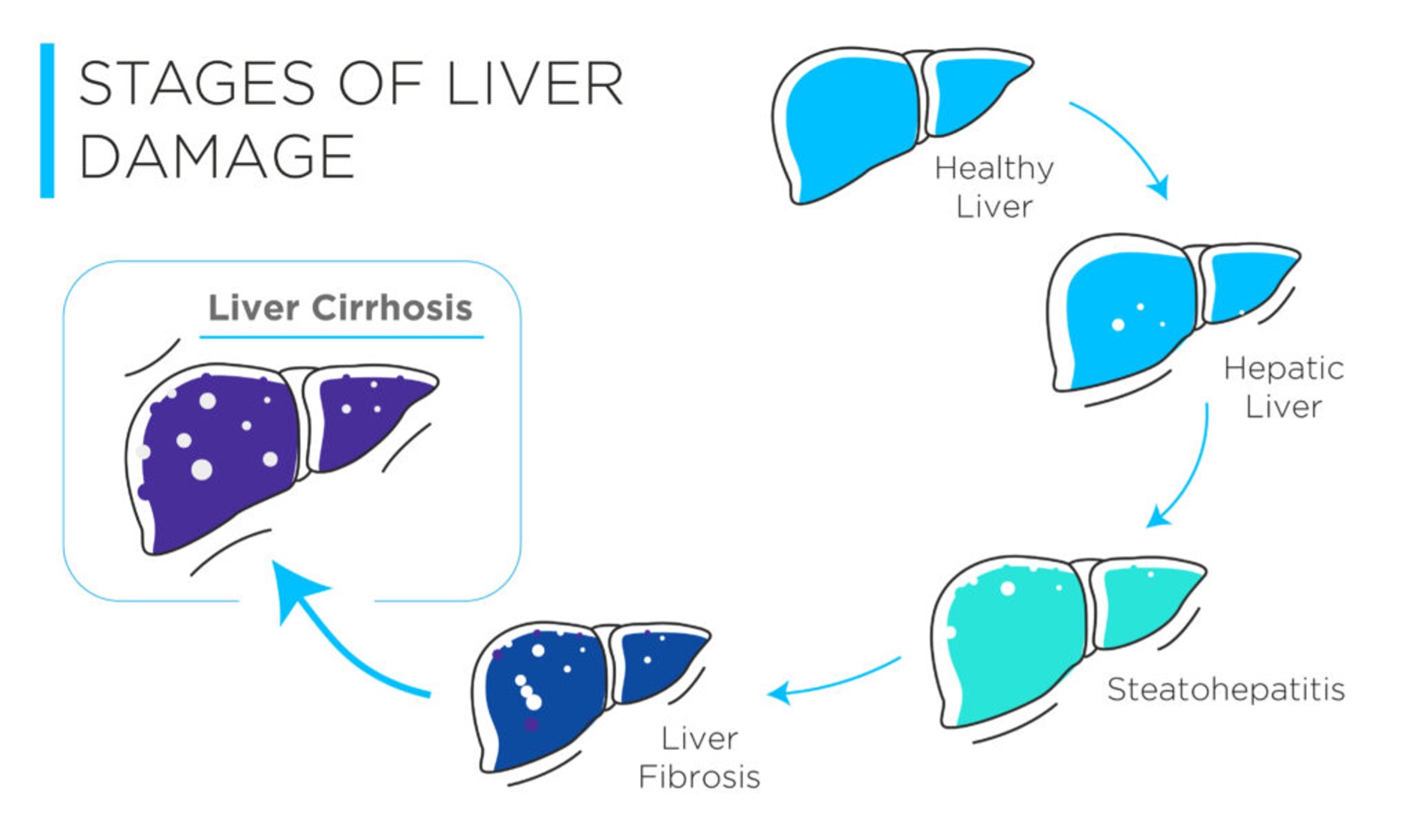
Figure 3: The stages of liver damage from healthy to diseased.
Chronic liver disease constitutes a spectrum of disorders (both alcohol, and non-alcohol related), that ultimately results in scarring of the liver, known as liver cirrhosis. In the initial stages, liver functions are largely preserved, and the patients often appear asymptomatic, making diagnosis difficult. However, as damage to the liver accumulates, cirrhosis can progress into a decompensated state, often accompanied by the occurrence of complications, such as liver failure, and cancer.
Studies have shown that SIBO has a significantly higher prevalence in those with liver cirrhosis (35.6%) than in healthy controls (3.6%), and also in patients with more severe disease: Child-Pugh class B or C (50%), compared to those with class A (19%) (20). In cirrhotic patients, the presence of SIBO predisposes the patients to bacterial translocation into the systemic circulation, which has a risk of causing infections such as bacterial peritonitis, and other complications such as hepatic encephalopathy (21). Learn more about the connection between gut dysbiosis and liver disease in our blog.
Parkinson’s Disease
Parkinson’s disease (PD) is a neurodegenerative disease that involves progressive motor/ movement issues and is thought to be caused by a loss of neurons in the substantia nigra of the brain, resulting in difficulty producing the neurotransmitter dopamine. PD is known to be associated with gastrointestinal symptoms such as nausea, bloating, and abdominal pain, which are thought to be caused by PD due to delayed gastric emptying, impaired gut motility, and defecatory dysfunction (22).
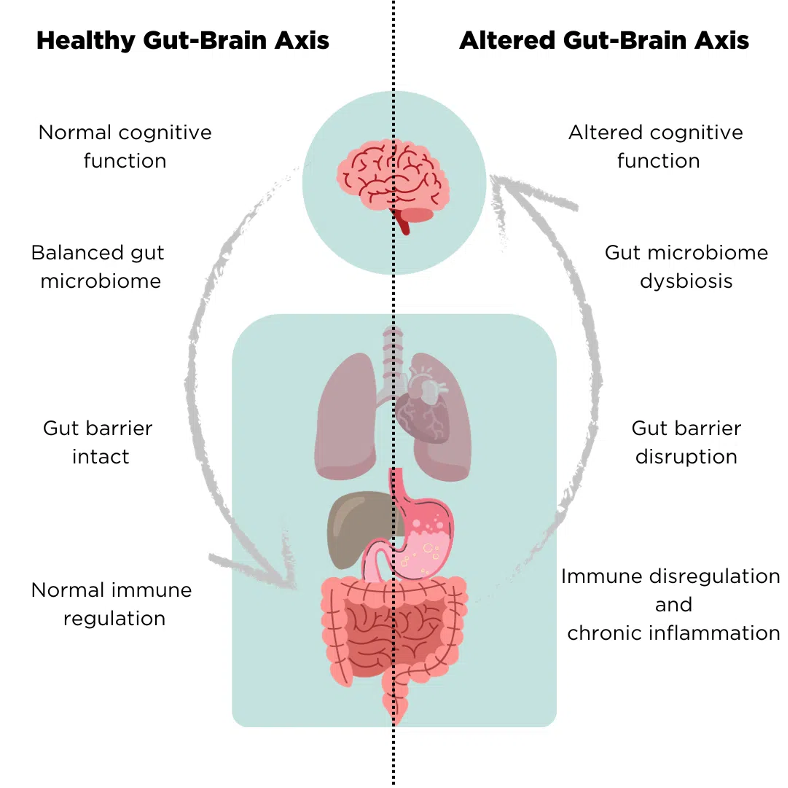
Figure 4: Differences between a healthy and altered gut-brain axis
Previous studies have reported a high prevalence of SIBO in PD patients (23). The presence of Helicobacter pylori and SIBO are both theorized to be able to trigger chronic proinflammatory changes in the body, which could exert a neurotoxic effect, as well as potentially alter the L-dopa response in PD patients (23). Previous studies have reported a prevalence of SIBO in PD patients between 54–67%, but these estimates were lowered in a large study of 103 PD patients, where 25.3% were SIBO-positive. It is worth noting that no control group was measured in this study for comparison. In this study, SIBO positivity was associated with worse motor function but was not associated with dopa-related motor response complications or with worse GI symptoms, suggesting that the GI symptoms are unlikely to be related to SIBO pathogenesis but instead be a direct result of PD.
Conclusion
The association of SIBO and other diseases is interesting, and it is not known whether the incidence of SIBO in these cases represents a risk factor for the development, or whether it is a consequence. It is possible that SIBO could lead to the systemic circulation of increasing key immunomodulatory and pro-inflammatory compounds around the body that could activate an inflammatory response in certain people who are perhaps predisposed to certain conditions. It is also possible that the incidence of SIBO can indicate the presence of an underlying chronic disease, and indicate dysbiosis of the gastrointestinal tract. Diagnosing SIBO patients in these cohorts, who could be extra-susceptible to SIBO, can help clinicians to lower the disease burden for these patients, as rifaximin and other antibiotic therapies can effectively treat SIBO (13,16).
Most of these studies utilized a lactulose breath test to diagnose the incidence of SIBO, demonstrating the power that breath analysis can provide when studying the interesting connection between the gut microbiome and the development of various diseases.
We can support clinical research by offering the analysis of the informative metabolites contained in breath, as well as portable hydrogen and methane breath analyzer devices that can diagnose SIBO anytime, anywhere, and support at-home collection of longitudinal data. This is ideal for incorporation into de-centralized clinical trial designs, better patient compliance, and non-invasive analysis. The device can also provide a cheaper alternative to the analysis of breath using laboratory-based techniques like TD-GC-MS. Beyond hydrogen and methane, hundreds of other informative compounds in the breath such as the short-chain fatty acids (SCFAs) have been linked to other diseases that can be analyzed using our Breath Biopsy OMNI® platform.
References
- Sender R, Fuchs S, Milo R. Revised Estimates for the Number of Human and Bacteria Cells in the Body. PLoS Biol. 2016 Aug 19;14(8):e1002533. doi: 10.1371/journal.pbio.1002533
- Dupont HL, Jiang ZD, Dupont AW, Utay NS. The Intestinal Microbiome In Human Health And Disease. Trans Am Clin Climatol Assoc. 2020;131:178–97. PMCID: PMC7358474
- Afzaal M, Saeed F, Shah YA, Hussain M, Rabail R, Socol CT, et al. Human gut microbiota in health and disease: Unveiling the relationship. Front Microbiol. 2022;13:999001. doi: 10.3389/fmicb.2022.999001
- Tripathi A, Debelius J, Brenner DA, Karin M, Loomba R, Schnabl B, et al. The gut-liver axis and the intersection with the microbiome. Nat Rev Gastroenterol Hepatol. 2018 Jul;15(7):397–411. doi: 10.1038/s41575-018-0011-z
- Zhu L, Baker SS, Gill C, Liu W, Alkhouri R, Baker RD, et al. Characterization of gut microbiomes in nonalcoholic steatohepatitis (NASH) patients: A connection between endogenous alcohol and NASH. Hepatology. 2013;57(2):601–9. doi: 10.1002/hep.26093
- Aron-Wisnewsky J, Clément K. The gut microbiome, diet, and links to cardiometabolic and chronic disorders. Nat Rev Nephrol. 2016 Mar;12(3):169–81. doi: 10.1038/nrneph.2015.191
- Jain T, Sharma P, Are AC, Vickers SM, Dudeja V. New Insights Into the Cancer–Microbiome–Immune Axis: Decrypting a Decade of Discoveries. Frontiers in Immunology. 2021;12. doi: 622064
- Kim S, Yin X, Prodhan MAI, Zhang X, Zhong Z, Kato I. Global Plasma Profiling for Colorectal Cancer-Associated Volatile Organic Compounds: a Proof-of-Principle Study. J Chromatogr Sci. 2019 May;57(5):385–96. doi: 10.1093/chromsci/bmz011
- Sachdev AH, Pimentel M. Gastrointestinal bacterial overgrowth: pathogenesis and clinical significance. Ther Adv Chronic Dis. 2013 Sep;4(5):223–31. doi: 10.1177/2040622313496126
- Bushyhead D, Quigley EMM. Small Intestinal Bacterial Overgrowth—Pathophysiology and Its Implications for Definition and Management. Gastroenterology. 2022 Sep 1;163(3):593–607. doi: 10.1053/j.gastro.2022.04.002
- Wu L, Hu J, Yi X, Lv J, Yao J, Tang W, et al. Gut microbiota interacts with inflammatory responses in acute pancreatitis. Therap Adv Gastroenterol. 2023 Oct 10;16:17562848231202133. doi: 10.1177/17562848231202133
- Hollemans RA, Hallensleben NDL, Mager DJ, Kelder JC, Besselink MG, Bruno MJ, et al. Pancreatic exocrine insufficiency following acute pancreatitis: Systematic review and study level meta-analysis. Pancreatology. 2018 Apr 1;18(3):253–62. doi: 10.1016/j.pan.2018.02.009
- Frost F, Kacprowski T, Rühlemann M, Bülow R, Kühn JP, Franke A, et al. Impaired Exocrine Pancreatic Function Associates With Changes in Intestinal Microbiota Composition and Diversity. Gastroenterology. 2019 Mar 1;156(4):1010–5. doi: 10.1053/j.gastro.2018.10.047
- El Kurdi B, Babar S, El Iskandarani M, Bataineh A, Lerch MM, Young M, et al. Factors That Affect Prevalence of Small Intestinal Bacterial Overgrowth in Chronic Pancreatitis: A Systematic Review, Meta-Analysis, and Meta-Regression. Clin Transl Gastroenterol. 2019 Sep 11;10(9):e00072. doi: 10.14309/ctg.0000000000000072
- Bures J, Cyrany J, Kohoutova D, Förstl M, Rejchrt S, Kvetina J, et al. Small intestinal bacterial overgrowth syndrome. World J Gastroenterol. 2010 Jun 28;16(24):2978–90. doi: 10.3748/wjg.v16.i24.2978
- Pan Y, Li J, Fan Z, Chen Y, Huang X, Wu D. New Insights into Chronic Pancreatitis: Potential Mechanisms Related to Probiotics. Microorganisms. 2024 Sep;12(9):1760. doi: 10.3390/microorganisms12091760
- Fridge JL, Conrad C, Gerson L, Castillo RO, Cox K. Risk Factors for Small Bowel Bacterial Overgrowth in Cystic Fibrosis. Journal of Pediatric Gastroenterology and Nutrition. 2007;44(2):212–8. doi: 10.1097/MPG.0b013e31802c0ceb
- Furnari M, De Alessandri A, Cresta F, Haupt M, Bassi M, Calvi A, et al. The role of small intestinal bacterial overgrowth in cystic fibrosis: a randomized case-controlled clinical trial with rifaximin. Journal of Gastroenterology. 2019 Mar 1;54(3):261–70. doi: 10.1007/s00535-018-1509-4
- Lewindon P, Robb T, Moore D, Davidson G, Martin A. Bowel dysfunction in cystic fibrosis: Importance of breath testing. Journal of Paediatrics and Child Health. 1998;34(1):79–82. doi: 10.1046/j.1440-1754.1998.00159.x
- De Lisle RC. Altered transit and bacterial overgrowth in the cystic fibrosis mouse small intestine. Am J Physiol Gastrointest Liver Physiol. 2007 Jul;293(1):G104-111. doi: 10.1152/ajpgi.00548.2006
- Grace E, Shaw C, Whelan K, Andreyev HJN. Review article: small intestinal bacterial overgrowth – prevalence, clinical features, current and developing diagnostic tests, and treatment. Alimentary Pharmacology & Therapeutics. 2013;38(7):674–88. doi: 10.1111/apt.12456
- C.-Y. Yang CSC GH Chen. Small-Intestinal Bacterial Overgrowth in Patients with Liver Cirrhosis, Diagnosed with Glucose H2 or CH4 Breath Tests. Scandinavian Journal of Gastroenterology. 1998 Jan;33(8):867–71. doi: 10.1080/00365529850171549
- Ghosh G, Jesudian AB. Small Intestinal Bacterial Overgrowth in Patients With Cirrhosis. Journal of Clinical and Experimental Hepatology. 2019 Mar 1;9(2):257–67. doi: 10.1016/j.jceh.2018.08.006
- Pfeiffer RF. Gastrointestinal dysfunction in Parkinson’s disease. Parkinsonism & Related Disorders. 2011 Jan 1;17(1):10–5. doi: 10.1016/j.parkreldis.2010.08.003
- Justich MB, Rojas OL, Fasano A. The Role of Helicobacter pylori and Small Intestinal Bacterial Overgrowth in Parkinson’s Disease. Semin Neurol. 2023 Aug;43(4):553–61. doi: 10.1055/s-0043-1771468