New methodology for VOC analysis from intubated mouse models
Remove background VOCs to obtain high quality samples
- New pre-clinical platform for breath VOC analysis enables rigorous animal model comparison
- The methodology integrates industry-standard volatile-capturing sorbent tubes with respiratory mechanics measurement equipment (flexi-Vent)
- Ability to accelerate the validation of VOCs identified from mouse models and their translation to clinical trials
INTRODUCTION
New methodology developed by experts in breath research to streamline sample collection from animal models
Exhaled breath contains volatile organic compounds (VOCs) that originate from biological processes in the body, and therefore have the potential to be utilized as biomarkers for clinical use. As breath biomarkers have been identified across a broad range of disease areas they are particularly promising for the broader adoption of next-generation non-invasive diagnostic and monitoring tools. However, the translation of breath tests to clinical use remains limited, which is partly due to the lack of consistent methodologies and quality controls across the breath research literature.
To address the challenges in clinical translation and expand the range of breath-based tests, a reliable animal model breath analysis method for pre-clinical studies is key. Establishing a method using inbred mice in controlled laboratory settings as a substitute for human breath can reduce the variability and challenges, allow for a better understanding of breath as a sampling medium using an animal model, and ultimately expediting the identification and validation of breath biomarkers for clinical use.
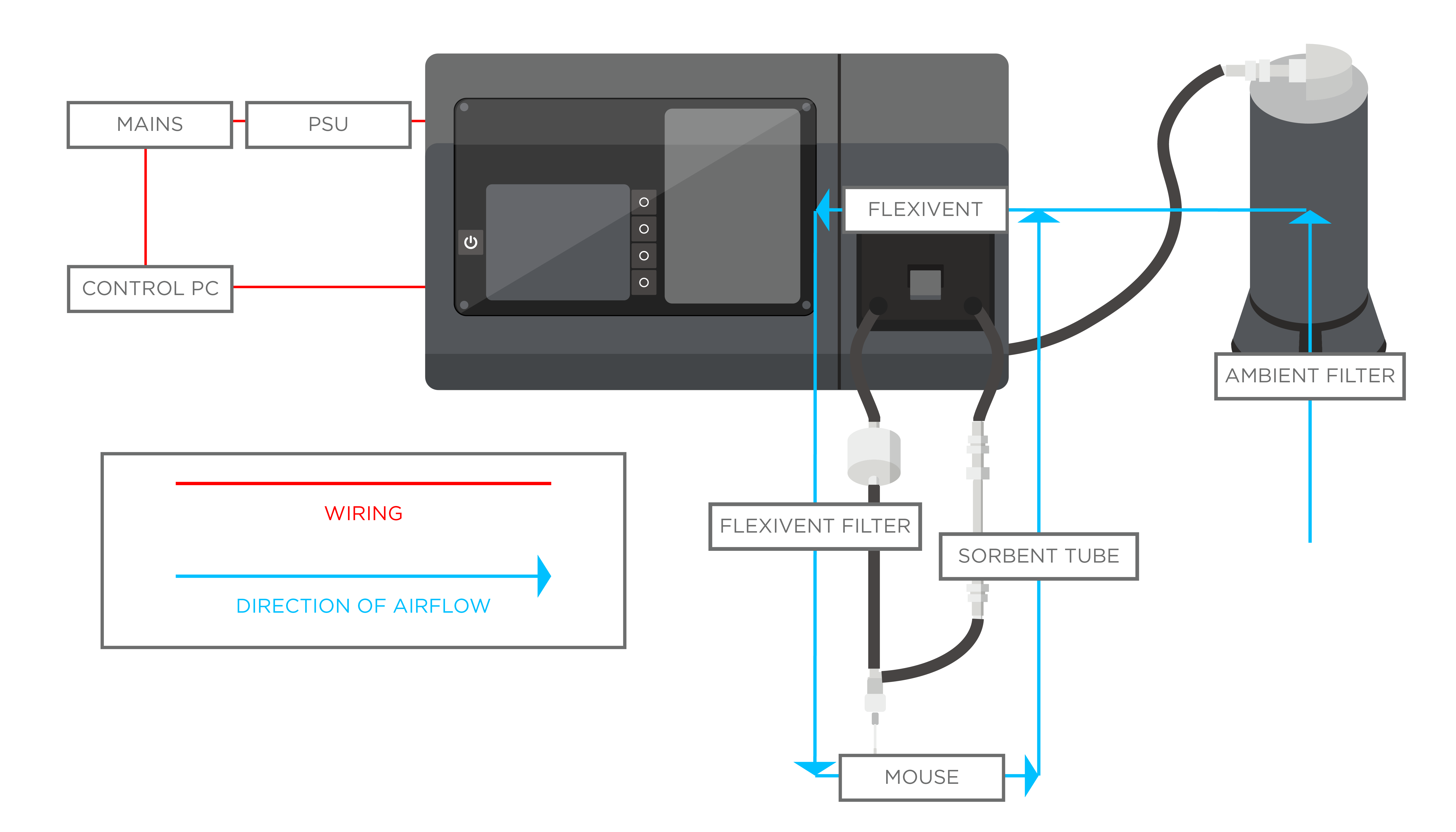
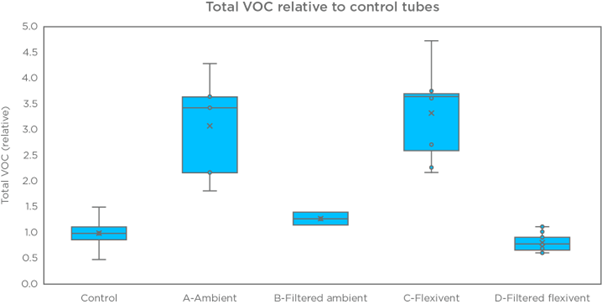
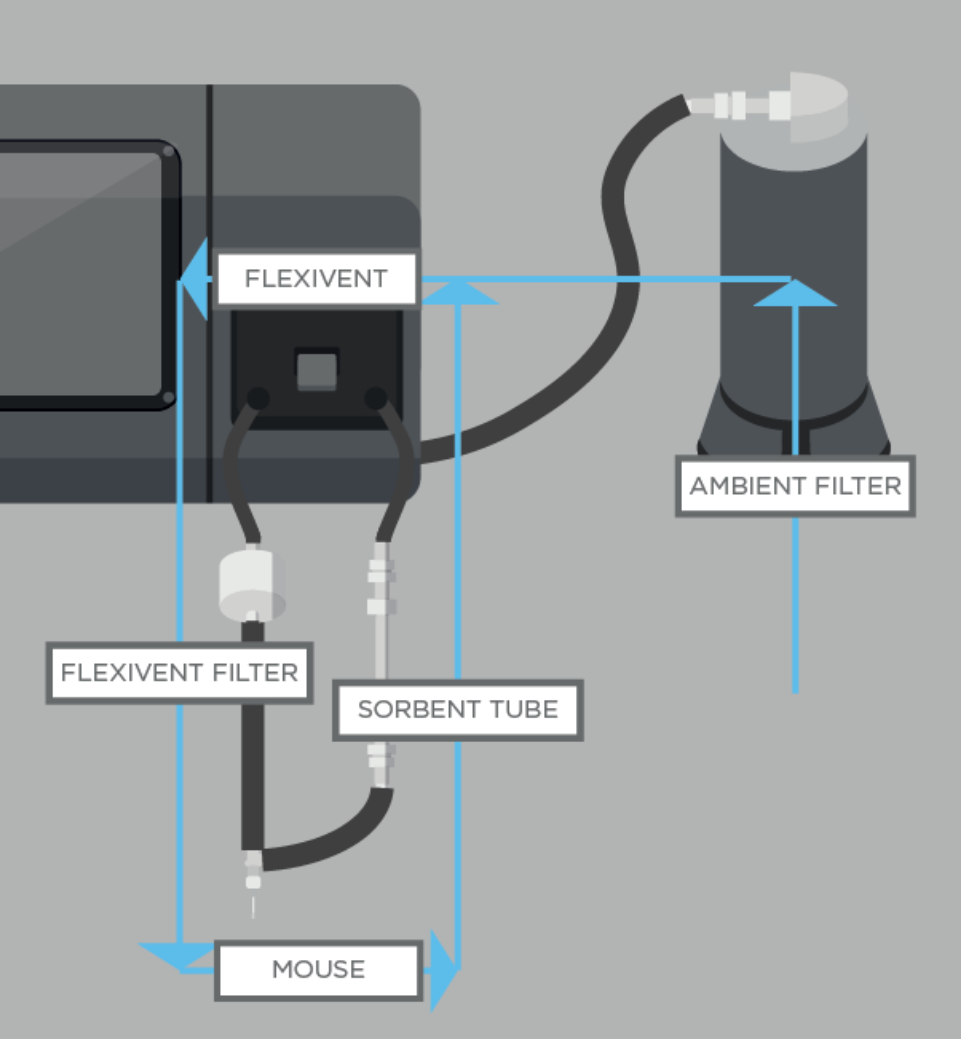
Several papers have previously aimed to study the breath of mouse models. In order to ensure that VOCs analyzed are truly exclusively from the breath, and to improve the signal-to-noise ratio, collection and analysis of breath from intubated mice can provide complementary data to advance the understanding of the mouse breath matrix.
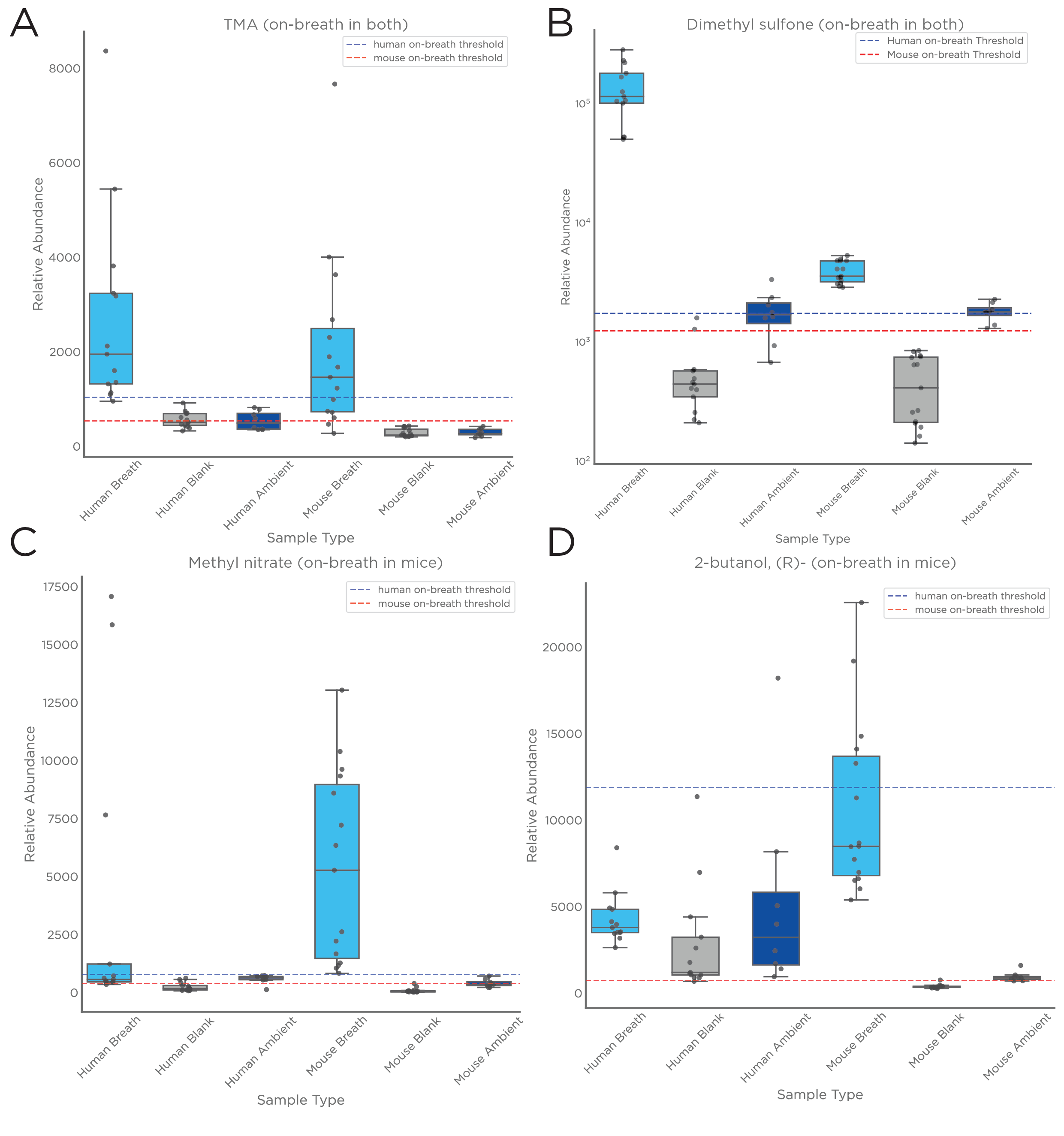
In a recent study, we developed a method for accurately characterizing the VOCs in the breath of healthy intubated mice using GC-MS, capable of superior control over the signal-to-noise ratios than is currently possible for human, and mouse breath. Finally, as a proof-of-principle and to assess the progress in data analysis quality, we compared the on-breath VOCs identified from intubated mouse models to the on-breath VOCs in human exhaled breath.
- Less Variability
A more controlled study, reduces the variability from inter-individuals, diet, and other environmental factors.
- Biomarker discovery
The reduced risk of false biomarker discovery accelerates the process of identifying potential biomarkers for validation in clinical trials.
- Contamination
Superior control over background contamination provides more confidence that the VOCs measured are genuinely breath-borne.
- Confidence
Analysis of chemical standards has provided high confidence VOC identification, allowing genuine comparison between mouse and human breath.
- comparable
Analysis of chemical standards has provided high confidence VOC identification, allowing genuine comparison between mouse and human breath.
- transferable
Three different metrics for VOC “on-breath” classification allow different stringency for distinguishing on-breath VOCs from background air, accommodating different study designs.
Flexivent system
VOC “on-breath” classification
Breath Analysis Toolbox: A comprehensive guide on how breath analysis can be used to aid GI microbiome research