Identifying breath biomarkers for colorectal cancer
Screening compliance is currently poor – a non-invasive test could save lives
| Publication information: D. F. Altomare et al., (2020) Chemical signature of colorectal cancer: case–control study for profiling the breath print, BJS Open, DOI: 10.1002/bjs5.50354
Disease Area: Colorectal cancer Application: Early detection Sample medium: Breath Products: ReCIVA® Breath Sampler Analysis approach: GC-MS Summary:
|
Effective screening for colorectal cancer would save lives by allowing earlier detection of cancerous growths at a time when they are more easily treatable. Unfortunately, current screening efforts are insufficient to achieve this, as they are often considered to be invasive and unpleasant. That means that even where a screening method has the ability to save lives its utility is limited, largely due to non-compliance.
A breath test capable of screening for the early stages of colorectal cancer would therefore be hugely advantageous. Providing a breath sample is an entirely non-invasive process so it could massively increase screening attendance. Additionally, establishing a volatile organic compound (VOC) biomarker on breath for colorectal cancer could lead to a test this is not only more palatable but also more sensitive than existing tests – capable of diagnosing cancer even earlier.
Methods & Results
In Altomare et. al’s paper they aimed ‘to evaluate the reliability of breath-testing for colorectal cancer screening and early diagnosis using an advanced breath sampler’. Their breath sampler of choice for this study was ReCIVA, which they used to collect samples from 83 patients with colorectal cancer and 90 non-cancer controls (judged as negative findings on a colonoscopy). After breath-based VOCs were captured on sorbent tubes by the ReCIVA they were subsequently desorbed and analyzed by GC-MS in the lab.
64 VOCs were detected in at least 50% of samples. In order to assess the discriminatory capacity of VOCs between the colorectal cancer group and the controls, 38 VOCs which appeared to differ between groups were assessed individually. 14 of these VOCs were found to significantly differ between groups, the most effective being tetradecane, ethylbenzene and methylbenzene, octanal, nonanal and decanal.
The success of this approach led the team to investigate more complex multivariate methods. This new model, combining results from the most effective of these VOCs, was notably able to detect early-stage cancer with an AUC of 98% and advanced cancer with an AUC of 93% (both rounded).
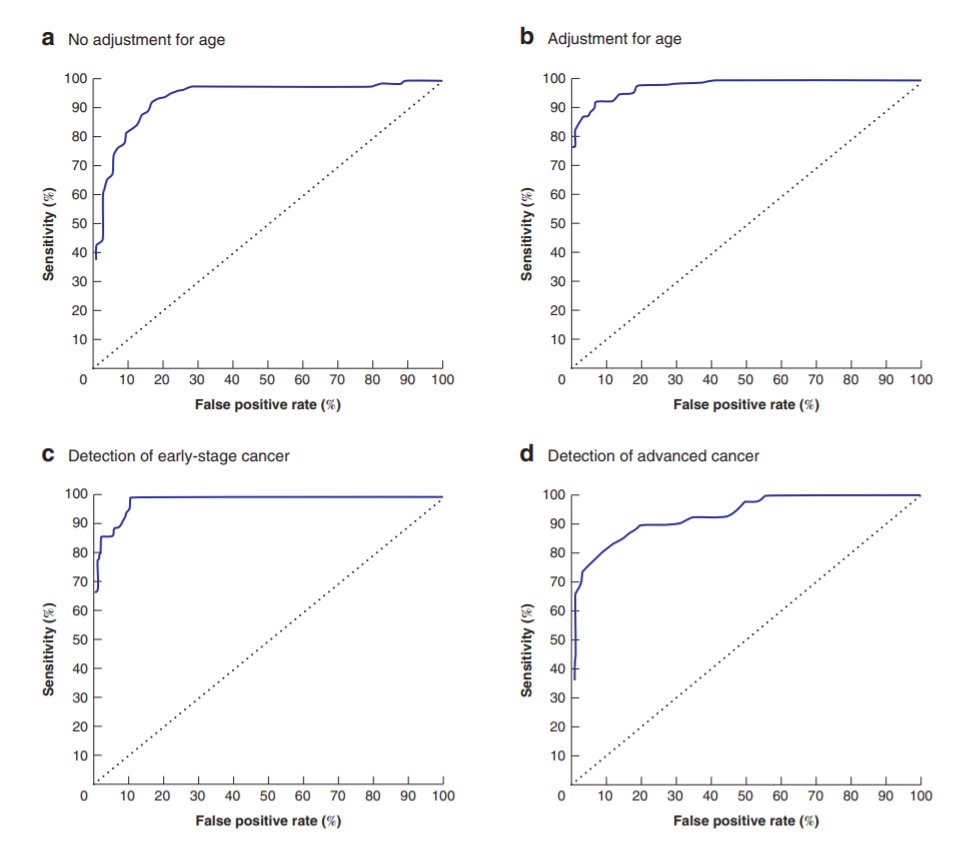
Figure 1. Receiver operating characteristic (ROC) curve analysis of the likelihood of discrimination for the model, with listed criteria (A-D)
Conclusions
These results seem to support the viability of breath as a screening option for colorectal cancer. Standardization is currently lacking across the breath field, which may explain the variety of different VOCs that other groups have identified as possible biomarkers in other recent studies. Both the ReCIVA Breath Sampler and Breath Biopsy® OMNI have been designed to provide reliable and reproducible results.
Owlstone Medical is hoping with these technologies to bring a new level of standardization to the field that will allow the results of studies like these to become more readily validated and advanced towards clinical applications.
Altomare et al. also note the far superior outputs of this study, compared to a similar previous effort that used a custom breath sampler, which found far fewer propsective VOC biomarkers. They state that in the previous study ‘The use of Tedlar bags for breath collection may also have provided an opportunity for extraneous VOC contamination’.
While we can’t yet explain the biological significance of the VOCs involved in carcinogenesis, it seems clear that some of those VOCs identified in this study, such as ethylbenzene and tetradecane, ‘correlate significantly with the diagnosis of colorectal cancer, irrespective of stage’. While further study is required, we can therefore speculate that their metabolic pathways could be closely linked to the process of carcinogenesis in the colon.
The next step for this work would be to undertake a larger, multi-center international study. This would be expensive work, but the goal after that study, would be to develop a simpler device, such as an e-nose, capable of translating these findings into the clinical context more cheaply, for use in screening programs.
If you’d to find out more about developing a breath test that could be used in the clinic to screen for colorectal cancer or other cancers, we have collated all of our resources on Breath Biopsy for in one place.