Differentiating Ferroptotic Lipid Peroxidation In Vivo: Volatile Organic Compounds as Breath Biomarkers
| Publication information: Yuta Matsuoka, Yoshinori Katsumata, Po-sung Chu, Rei Morikawa, Nobuhiro Nakamoto, Kohto Iguchi, Ken Takahashi, Tadayuki Kou, Ryo Ito, Kojiro Taura, Shujiro Yazumi, Hiroaki Terajima, Genki Ichihara, Yuki Muramoto, Kazuki Sato, Rae Maeda, Naoya Toriu, Motoko Yanagita, Masaki Tajima, Sidonia Fagarasan, Ken-ichi Yamada, Yuki Sugiura. Volatile oxidized lipids generated via metal-dependent lipid peroxidation of ω-6 PUFAs are breath biomarkers for monitoring ferroptosis in vivo.
Application: Biomarker Development Summary:
|
Introduction
Since its first definition in 2012 (1) , ferroptosis has been recognized as a unique form of cell death, distinct from apoptosis, and is defined by its reliance on iron to drive lipid peroxidation within cell membranes. This process has quickly gained attention for its broad implications in various diseases, ranging from cancers to neurodegenerative disorders, ischemia-reperfusion injury, kidney disease, organ fibrosis, and cardiovascular conditions (2).
The role of ferroptosis varies significantly across these diseases. In the context of neurodegeneration, Type II diabetes, and kidney dysfunction, it tends to exacerbate disease progression, representing an unfavorable outcome. However, ferroptosis can also play a beneficial role in targeting cancer cells, making it a potential therapeutic target in certain malignancies.
Ongoing research has only begun to unravel the complex roles of ferroptosis, with scientific publications on the subject expanding by approximately 75% year over year. Amongst the major challenges remaining are reliable, non-invasive methods to detect ferroptosis in clinical settings (3). Recently, promising work by Matsuoka and colleagues suggests that volatile organic compounds (VOCs) in breath may serve as biomarkers for ferroptosis, potentially offering a novel, non-invasive detection method for tracking this unique cell death pathway.
Volatile organic compounds (VOCs) are produced in cells through both enzyme-driven and spontaneous metabolic reactions, and their emission can vary with changes in cellular state. One key process that generates VOCs is lipid peroxidation, where cell membrane lipids are oxidized by reactive oxygen species (ROS). This mechanism is notably active in ferroptosis.
While specific lipid peroxidation products linked to ferroptosis have been identified (4), it remains unclear how sensitively and specifically breath-based VOCs can report on lipid peroxidation events that are unique to ferroptosis.
Dr. Yuta Matsuoka from Kyoto University recently presented this study at the Breath Biopsy Conference 2024. You can watch Dr. Matsuoka’s talk, as well as the rest of the presentations from the conference here.
Methods
This work comprised in vitro work, cell culture work, in vivo and in homine work. For the in vitro work, poly-unsaturated fatty acids (PUFAs), dihydrochloride and hemin were mixed in solid-phase microextraction (SPME) vials. These were then incubated at 37oC for 2 hours and analyzed via SPME-GC/MS. For cell culture work, HepG2 cells were treated with an apoptosis inducer (STS) and/or a ferroptosis inhibitor (Lip-1). Following a 24-hour incubation these cells and culture media were transferred to an SPME vial and analyzed via SPME-GC/MS.
For the in vivo work, APAP-ALF mice and MASH mice were generated. VOCs were analyzed via TD-GC/MS. For the in homine work, patients were recruited from Keio University Hospital and Kitano hospital. Healthy individuals had no comorbidities (hypertension, diabetes or active lung disease). Samples were collected via the ReCIVA breath sampler connected to a CASPER portable air supply and analyzed by TD-GC/MS.
Results
A VOC library was generated from the products of lipid peroxidation of various poly-unsaturated fatty acids (PUFAs), defined as VOCs that demonstrate a two-fold increase under conditions of lipid peroxidation over controls. A total of 48 VOCs were detected arising from the oxidation of 4 ω-6 and ω-3 PUFAs, with products from ferroptosis resulting in a wider diversity of measured VOCs, including vinyl alcohols (Figure 1). A reaction schema demonstrating the involvement of iron in the generation of these VOCs is shown in Figure 2.
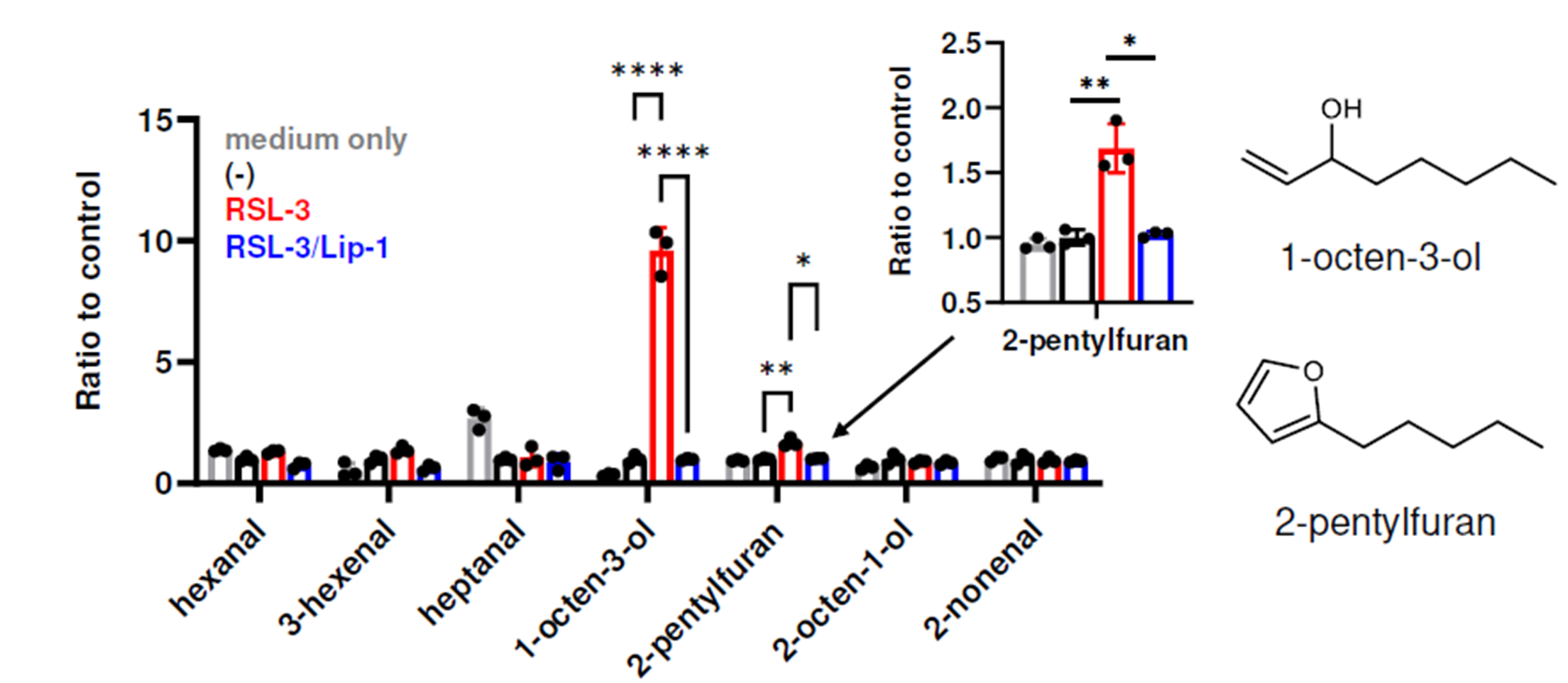
Figure 1 – The production of 1-octen-3-ol and 2-pentyl-furan are significantly elevated over baseline when incubated in ferroptotic conditions (RSL-3) and this can be acutely blocked through the addition of inhibitors of ferroptosis (Lip-1). Image from Matsuoka et al., 2024.
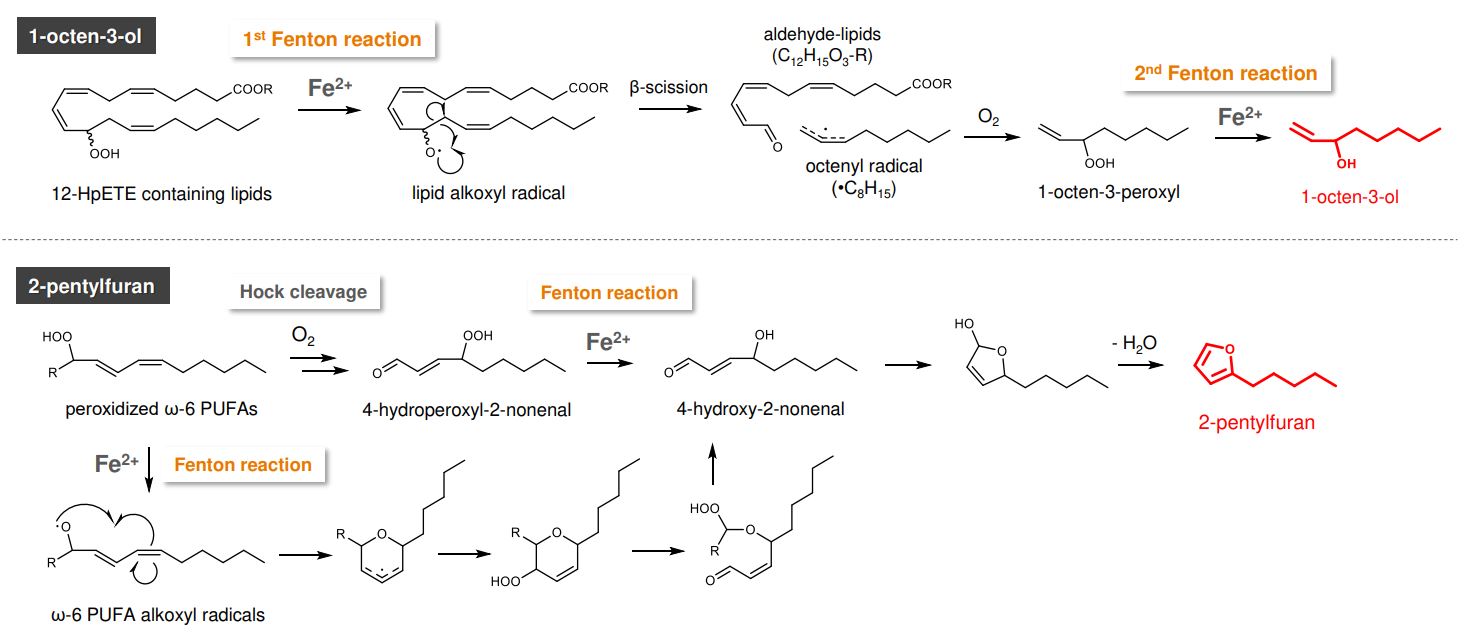
Figure 2 – Proposed generation mechanisms of 1-octen-3-ol and 2-pentylfuran, with the requirements for iron-catalysed reaction steps highlighted. Image from Matsuoka et al., 2024.
To validate this VOC library’s relevance, VOCs released from HepG2 cells treated with RSL-3, a ferroptosis inducer, were analyzed. Seven VOCs, including 1-octen-3-ol and 2-pentylfuran, aligned with those in the library and were confirmed to be ferroptosis-specific, as their generation was inhibited by Lip-1, a ferroptosis inhibitor. Using radiolabeled ω-6 PUFAs in HepG2 cells further validated this pathway, confirming the production of deuterium-labeled 1-octen-3-ol and 2-pentylfuran during ferroptosis.
Subsequently, mice with acetaminophen (APAP)-induced acute liver failure—a condition associated with extensive ferroptosis in liver cells—were examined (Figure 3). Both headspace VOC analysis of liver samples and breath analysis from the mice in sealed cages demonstrated elevated levels of 1-octen-3-ol and 2-pentylfuran. Administration of N-acetylcysteine (NAC) reduced these VOCs, and similar VOC increases were observed in an alternative liver failure model, affirming the consistency of findings across liver injury types.
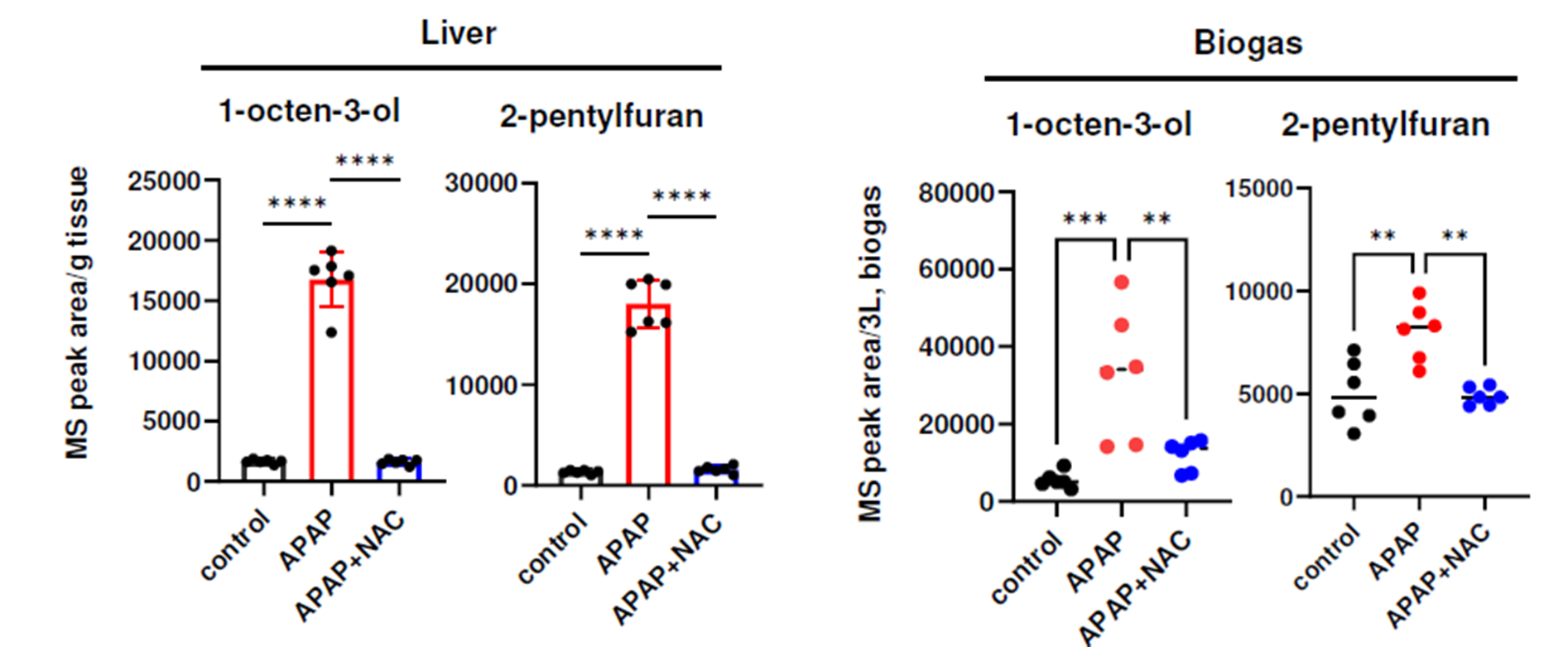
Figure 3 – Elevations in 1-octen-3-ol and 2-pentylfuran were elevated in a mouse model of liver failure, both in the headspace of liver samples, as well as in-breath. Image from Matsuoka et al., 2024.
Finally, VOCs were collected via the ReCIVA® Breath Sampler from 48 healthy controls, 44 patients with MASLD without cirrhosis and 10 patients with MASLD with cirrhosis. These data demonstrated an increase in 1-octen-3-ol and 2-pentylfuran in patients with MASLD compared to controls, and 2-pentylfuran being significantly elevated in cirrhotic patient’s vs non-cirrhotic patients (Figure 4).
These VOCs also correlated significantly with disease severity markers: 1-octen-3-ol with plasma ALT and 2-pentylfuran with fibrotic markers (FIB-4 index, M2BPGi, and ALBI score). The combination of these VOCs with standard clinical markers provided discrimination between healthy patients, MASLD patients with cirrhosis, and MASLD patients without cirrhosis with an AUC>0.9.
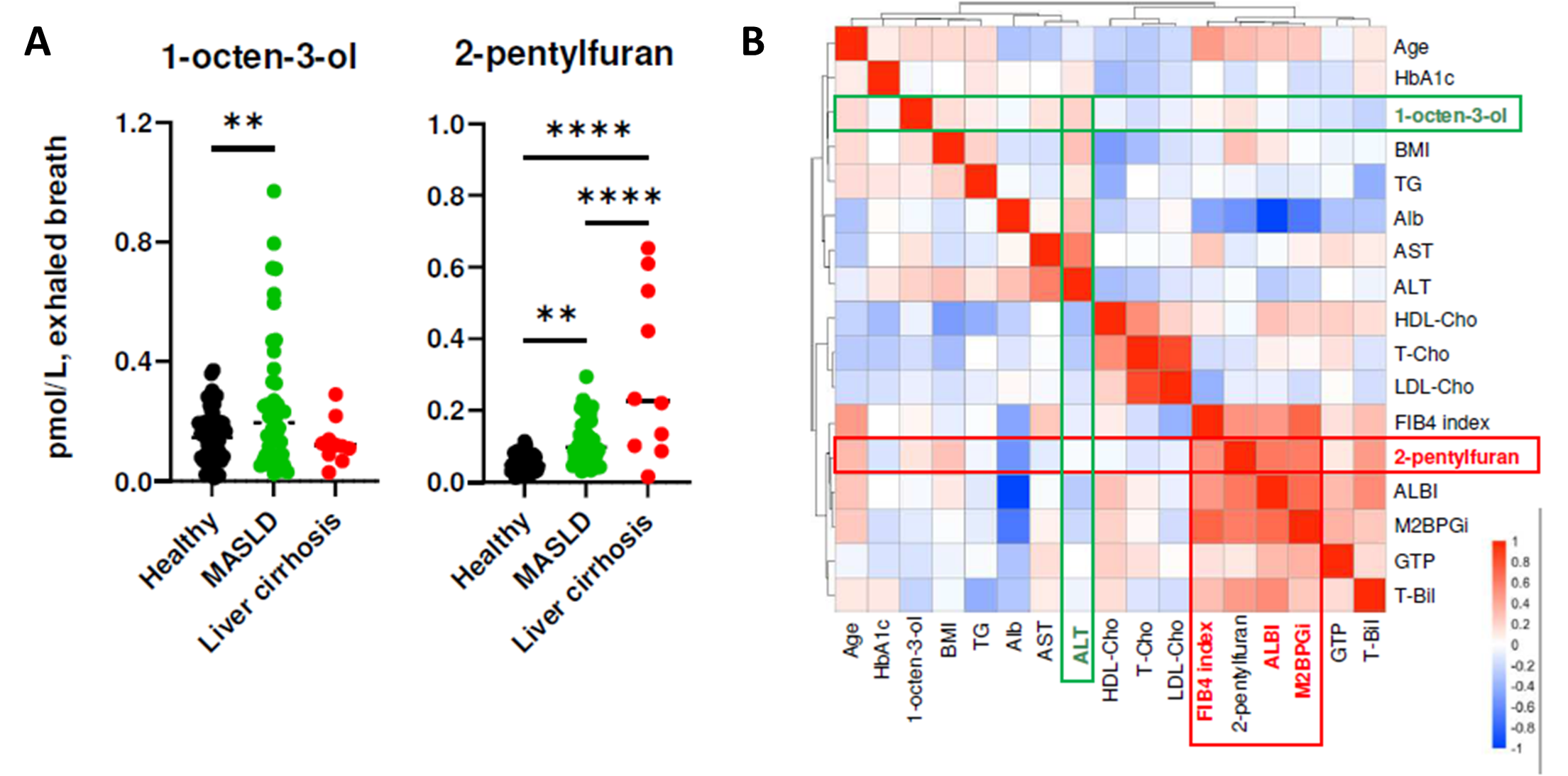
Figure 4 – Demonstrating A) the increase in 1-octen-3-ol and 2-pentyfuran in patients with MASLD compared to healthy controls, with 2-pentylfuran demonstrating a further increase in MASLD patients with cirrhosis. B) the correlation between 1-octen-3-ol and 2-pentyfuran with markers of liver health, as well as fibrosis. Image from Matsuoka et al., 2024.
Discussion
Together this work highlights the ability of breath VOCs to report on specific biochemical pathways, here, on ferroptotic lipid peroxidation over other forms of lipid peroxidation. This is demonstrated at a process level via organic chemistry and further confirmed by in vitro radiolabeled experiments. The potential application of these specific signatures is illustrated in the context of liver disease, first through various mouse models and ultimately in a human population.
The potential impact of VOCs in this disease area is promising, with levels of 1-octen-3-ol and 2-pentylfuran serving as a non-invasive reporter, closely correlating both with standard clinical markers of liver function, as well as markers of fibrosis. The effectiveness of breath VOCs is highlighted when combined with standard clinical markers, achieving an area under the curve (AUC) greater than 0.9 for distinguishing healthy patients from those with MASLD, as well as differentiating MASLD patients based on the presence of cirrhosis.
Interestingly, while 2-pentylfuran levels showed a stepwise increase across groups, from healthy controls to patients with MASLD and then to patients with liver cirrhosis, 1-octen-3-ol increased from healthy controls to MASLD patients but then decreased in those with cirrhosis. There are a few potential drivers which may be causing this behavior. As noted in the study, the resistance to ferroptosis during the progression to cirrhosis could explain the reduction in 1-octen-3-ol levels (5).
Alternatively, this may reflect changes in substrate availability, given that the production of 1-octen-3-ol is believed to depend on arachidonic acid, which decreases in cirrhosis patients (6). Conversely, the increased levels of 2-pentylfuran in cirrhosis patients may be due to enhanced access to dietary linoleic acid (7).
These discrepancies between in vitro studies and in vivo observations, along with the potential influence of confounding variables, underscore the challenges currently faced in breath research. Owlstone Medical is developing a database, the VOC Atlas, to enhance understanding of both the biochemical relevance of breath biomarkers as well as the potential confounders affecting their interpretation. An examination of this database indicates that 2-pentylfuran is present in the breath of healthy individuals. Below is a table of compounds associated with liver cirrhosis that are included in the Atlas. Sign up to find out more about how the VOC Atlas can help your research.

Table 1. List of compounds associated with liver cirrhosis reported more than twice in the literature that are found in the Breath Biopsy VOC Atlas.
References
- Dixon, S. J. et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell 149, 1060–1072 (2012). DOI: 10.1016/j.cell.2012.03.042
- Li, J. et al. Ferroptosis: past, present and future. Cell Death Dis. 11, 88 (2020). DOI: 10.1038/s41419-020-2298-2
- Liu, Q., Zhao, Y., Zhou, H. & Chen, C. Ferroptosis: challenges and opportunities for nanomaterials in cancer therapy. Regen. Biomater. 10, rbad004 (2023). DOI: 10.1093/rb/rbad004
- Kagan, V. E. et al. Oxidized arachidonic and adrenic PEs navigate cells to ferroptosis. Nat. Chem. Biol. 13, 81–90 (2017). DOI: 10.1038/nchembio.2238
- Chen, J., Li, X., Ge, C., Min, J. & Wang, F. The multifaceted role of ferroptosis in liver disease. Cell Death Differ. 29, 467–480 (2022). DOI: 10.1038/s41418-022-00941-0
- Safaei, A. et al. Metabolomic analysis of human cirrhosis, hepatocellular carcinoma, non-alcoholic fatty liver disease and non-alcoholic steatohepatitis diseases. Gastroenterol. Hepatol. Bed Bench 9, 158 (2016). PMCID: PMC4947130
- Zhu, T., Lu, X.-T., Liu, Z.-Y. & Zhu, H.-L. Dietary linoleic acid and the ratio of unsaturated to saturated fatty acids are inversely associated with significant liver fibrosis risk: A nationwide survey. Front. Nutr. 9, (2022). DOI; 10.3389/fnut.2022.938645
Catch up on the presentations from the Breath Biopsy Conference 2024
