Limonene as a Diagnostic Breath Test for Cirrhosis
Detecting liver disease is challenging but breath limonene could hold the solution.
| Publication information: Ferrandino G, Ricciardi F, Murgia A, Banda I, Manhota M, Ahmed Y, Sweeney K, Nicholson-Scott L, McConville L, Gandelman O, Allsworth M. Exogenous Volatile Organic Compound (EVOC®) Breath Testing Maximizes Classification Performance for Subjects with Cirrhosis and Reveals Signs of Portal Hypertension. Biomedicines. 2023 Nov 1;11(11):2957. DOI: 10.3390/biomedicines11112957
Disease Area: Liver disease (cirrhosis, NAFLD, NASH) Application: Early detection, progression monitoring Sample medium: Breath Products: ReCIVA® Breath Sampler, CASPER®, Portable Air Supply, Breath Biopsy®, EVOC® Probes Summary:
|
Globally, levels of liver disease are rising rapidly, with as many as 3 in 10 adults and 1 in 10 children affected, in some countries (1). Unfortunately, despite their increasing incidence, detecting liver diseases, such as non-alcoholic fatty liver disease (NAFLD) and non-alcoholic steatohepatitis (NASH) as well as more advanced cirrhosis, remains challenging.
Detection currently is expensive and relies heavily upon invasive methods such as blood and tissue biopsy. Further, these tests largely reflect liver damage and not current liver function – meaning that damage has to accumulate first before liver disease can be reliably diagnosed. A non-invasive method suitable for screening would enable earlier detection and would be hugely beneficial to get patients access to treatment earlier to help prevent the accumulation of potentially irreversible damage to the liver.
Three prior publications have established the relevance of breath limonene for the detection of liver disease. Fernandez del Rio et al. initially linked limonene with liver diseases, by comparing levels of limonene on the breath of liver cirrhosis patients pre- and post-liver transplant (Figure 1) (2). In a follow-up paper O’Hara M. E. et al, demonstrated that limonene levels moderately increased in patients with hepatocellular carcinoma (HCC) with underlying cirrhosis (3). Ferrandino et al. then demonstrated that utilizing limonene as an Exogenous Volatile Organic Compound (EVOC) probe could result in different levels of limonene in the breath, capable of differentiating between healthy and cirrhotic individuals with 75% accuracy (4).
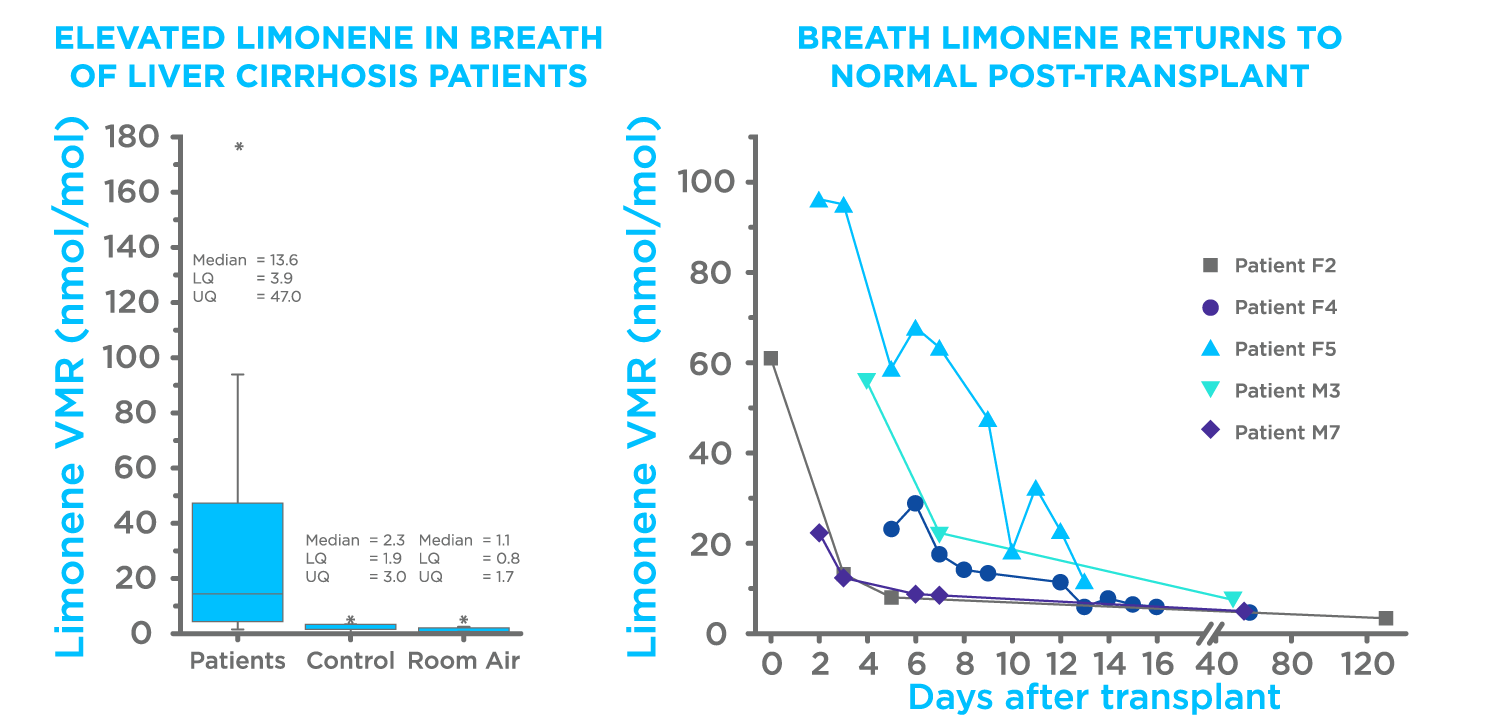
Figure 1. Results from Fernandez del Rio et al (2).
This study investigated the to investigate whether a dynamic approach to breath testing, i. e. measuring limonene at multiple time points after administration, could improve the diagnostic performance of the test for cirrhosis.
Dynamic Breath Testing with EVOC probe Limonene for Liver Disease
29 subjects with cirrhosis and 29 controls were enrolled in this study, and breath samples were taken using Breath Biopsy® before and at different time points after the administration of 100 mg of limonene. Levels of limonene in the breath samples were measured using our OMNI® platform.
The ingestion of 100 mg limonene induced a spike in breath of >100-fold the baseline levels in all subjects. More than 90% of the subjects showed a limonene maximal breath amount (Cmax) within 20 and 40 min (Tmax). The investigated time course in a semi-logarithmic presentation showed single-phase exponential decay of breath limonene with first-order kinetics (R2 > 0.8) in >90% of the subjects (Figure 1A). Comparisons of all the participants showed that subjects with cirrhosis had higher levels of limonene in the breath at each tested timepoint (p < 0.001), with the cirrhosis group presenting with a higher Cmax and bioavailability (Figure 2). These data indicate that the effect of cirrhosis on limonene exhalation kinetics mirrors the pharmacokinetic (PK) alterations induced by cirrhosis on drugs with high hepatic extraction, also known as flow-limited (5,6).
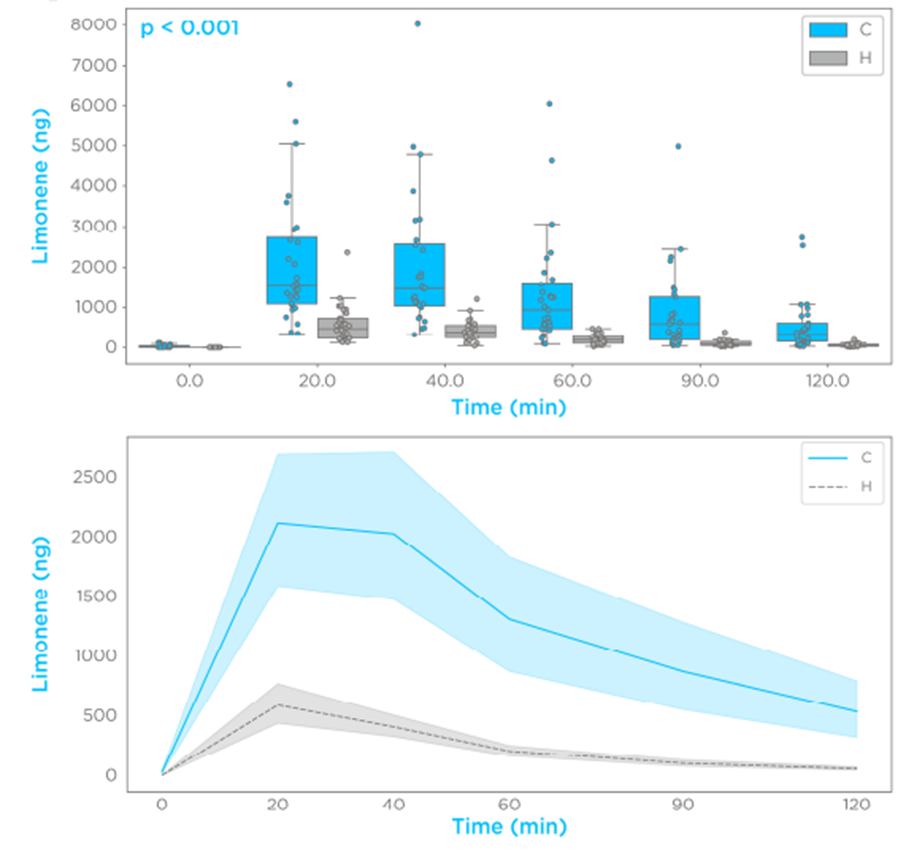
Figure 2 – Limonene exhalation shows first-order kinetics and increased bioavailability in subjects with cirrhosis.
The timepoint with the best performing diagnostic performance was shown to be at 60 minutes post-limonene ingestion (Figure 3), with an AUROC of 0.91 ± 0.07, a specificity of 0.9 ± 0.06, and a sensitivity of 0.83 ± 0.07. These findings suggest that a dynamic limonene breath test is a potential high-performing diagnostic test for cirrhosis.
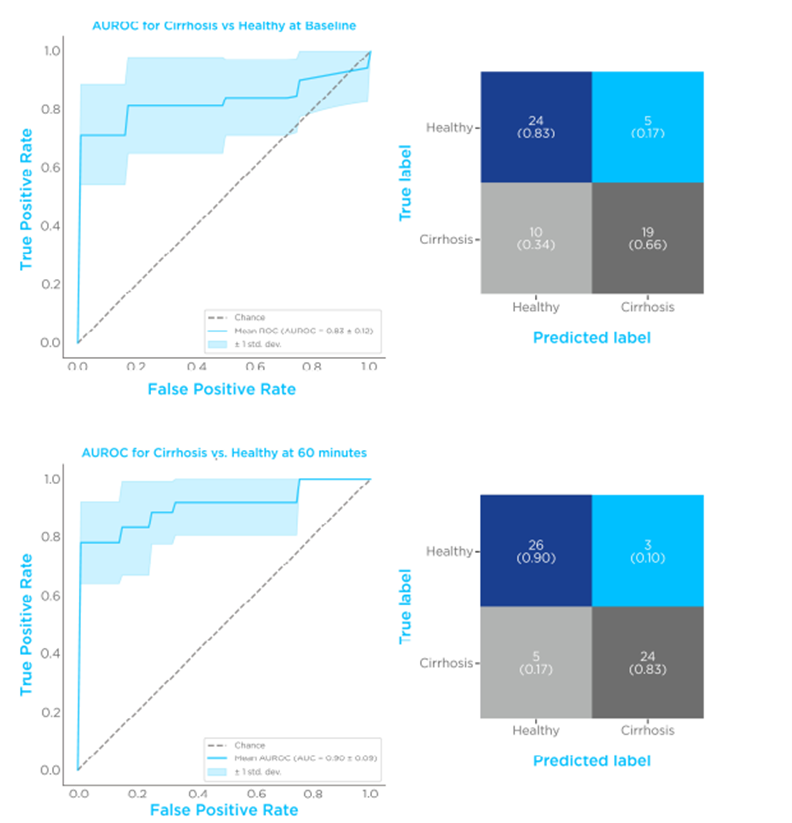
Figure 3 – Limonene classification performances. Comparison of breath limonene levels of control vs. cirrhosis. The top set shows an ROC plot and confusion matrix before limonene administration, the bottom set is 60 minutes after limonene administration.
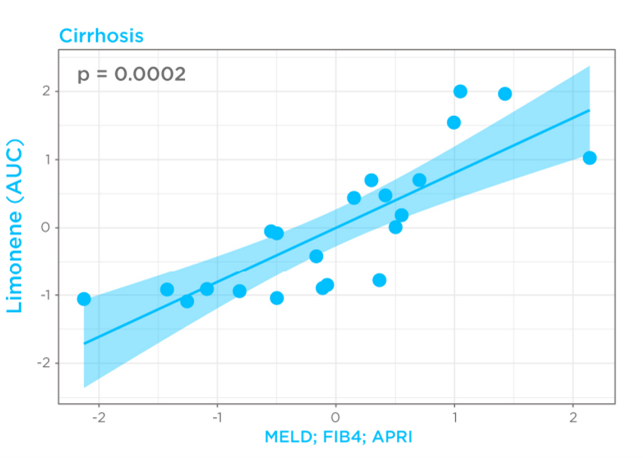
Given that cirrhosis-induced alterations in limonene kinetics in the breath appeared to mimic drugs with high hepatic extraction, breath limonene AUC could therefore be used as a proxy of bioavailability in the cirrhosis group. Known Liver cirrhosis indicators such as the MELD score (Model for End-Stage Liver Disease) for disease severity, and FIB4 (Fibrosis-4) and APRI (AST to Platelet Ratio Index) for risk of advanced fibrosis, were then correlated with breath limonene. In the cirrhosis group, breath limonene AUC showed a significant correlation with the scoring systems (p = 0.0002), with the MELD score being shown to be the main parameter contributing to the correlation (Figure 4).
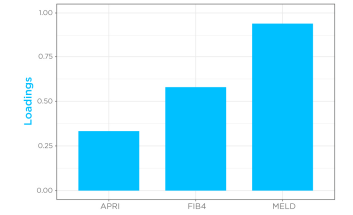
Figure 4 – Correlation of limonene bioavailability with MELD, FIB4, and APRI in the cirrhosis group. D, FIB4, and APRI in the cirrhosis group.
The paper also described an interesting case study, where the proposed limonene dynamic breath test was able to correctly re-categorize a patient who was accidentally sorted in the control group, who had cirrhosis – as confirmed by ultrasound.
With the high levels of sensitivity and specificity achieved in this study, this demonstrates that a dynamic limonene breath analysis could maximize the diagnostic performance of a breath test for cirrhosis. As breath analysis is non-invasive and well tolerated by patients this approach could complement, or even potentially replace current clinical diagnostic techniques due to the suitability for early detection and implementation into primary care.
If you are interested in the potential of breath analysis in tackling cardiometabolic diseases, the full study is available to read on our website. You can also get in touch with our team of breath experts to discuss expediting your biomarker discovery workflow.
References
- Definition & Facts of NAFLD & NASH: https://www.niddk.nih.gov/health-information/liver-disease/nafld-nash/definition-facts
- Fernandez de Rio et al. (2015) Volatile Biomarkers in Breath Associated With Liver Cirrhosis — Comparisons of Pre- and Post-liver Transplant Breath Samples EBioMedicine 2(9), p1243-1250, DOI: 10.1016/j.ebiom.2015.07.027
- M E O’Hara et al. (2016) Limonene in exhaled breath is elevated in hepatic encephalopathy J. Breath Res. DOI: 10.1088/1752-7155/10/4/046010
- Ferrandino et al. (2020) Breath Biopsy Assessment of Liver Disease Using an Exogenous Volatile Organic Compound—Toward Improved Detection of Liver Impairment Clinical and Translational Gastroenterology 11:e00239. DOI: 10.14309/ctg.0000000000000239
- Krähenbühl S, Reichen J. Pharmacokinetics and Pharmacodynamics in Cirrhosis. Medicine. 2002 Nov 1;30(11):24–7. DOI: 10.1383/medc.30.11.24.28446
- Duthaler U, Bachmann F, Suenderhauf C, Grandinetti T, Pfefferkorn F, Haschke M, et al. Liver Cirrhosis Affects the Pharmacokinetics of the Six Substrates of the Basel Phenotyping Cocktail Differently. Clin Pharmacokinet. 2022 Jul;61(7):1039–55. DOI: 10.1007/s40262-022-01119-0
Quick Start Guide: Everything you need to know about how breath analysis can be used in liver disease research