Discover the link between SIBO and Rosacea
Dysbiosis of the gut microbiome has been associated with the development of many diseases. Small Intestinal Bacterial Overgrowth (SIBO), as diagnosed by hydrogen methane breath testing was shown to be significantly higher in rosacea patients than controls, and treating SIBO resulted in a dramatic improvement of rosacea lesions.
| Publication information: Parodi et al. Small Intestinal Bacterial Overgrowth in Rosacea: Clinical Effectiveness of Its Eradication. Clinical Gastroenterology and Hepatology. 2008 Jul 1;6(7):759–64. DOI: 10.1016/j.cgh.2008.02.054.
Aims: To better understand the role of small intestinal bacterial overgrowth (SIBO) in rosacea, and the clinical effectiveness of its eradication. Sample medium: Breath Analysis approach: GC (HMBT) Summary:
|
Introduction to SIBO and rosacea.
The microbiome is well known to be a key regulator of the immune system, and dysbiosis of the gut microbiome has been associated with the development of many diseases such as inflammatory bowel disease (IBD), metabolic-associated steatohepatitis (MASH), metabolic dysfunction-associated fatty liver disease (MAFLD), kidney disease, and cancer (1–7). This includes several skin diseases such as atopic dermatitis, psoriasis, and acne through dynamic and complex mechanisms (8).
Rosacea is a long-term skin condition characterized by reddened skin and a face rash. It is more common in women, but the symptoms can be worse in men. Rosacea is an inflammatory condition that can present in many different ways, with four distinct reoccurring presentations: flushing, erythrosis (a red or purplish color of the skin resulting from abnormalities in the blood vessels), papulopustules (pus-filled blemishes, and red, swollen bumps), and phymata (the most severe, rare presentation of disfiguring soft-tissue and sebaceous gland growth and/or thickening).
These presentations are very heterogeneous in patients, and several complex environmental and endogenous factors are thought to be involved in the pathogenesis of rosacea, including genetic predisposition, UV light, heat, immune disruption, and microbiome dysbiosis (9,10). Patients with rosacea often report gastrointestinal symptoms such as bloating and abdominal pain. Therefore it was hypothesized that these symptoms could be caused by a link between the skin and the activity of the gut microbiome.
A significant number of the volatile compounds detectable in exhaled breath are thought to originate from the gut microbiome, and therefore provide an interesting method to investigate the link between dysbiosis and skin disease (11). One interesting potential mechanism of the association came from a case study of one patient who experienced complete remission of their rosacea after their gut transit time was altered (12). Abnormal alterations in gut transit time and intestinal motility are known to be a risk factor for Small Intestinal Bacterial Overgrowth (SIBO) (13).
Therefore, Parodi et al. investigated the link between SIBO and rosacea by combining hydrogen and methane breath testing with dermatological analysis and gastrointestinal symptom reporting (14). The aims of this study were to assess the prevalence of SIBO in rosacea patients and evaluate the effects of treating SIBO on rosacea symptoms.
Methods
A total of 113 rosacea patients (82 women, 31 men) and 60 healthy, sex- and age-matched controls (40 women, 20 men) were enrolled in this study. All patients had their baseline biochemical and stool analyses performed prior to the study and completed a hydrogen and methane breath test (HMBT) using both a lactulose and glucose test substrate to measure the baseline breath composition. Two independent dermatologists evaluated the clinical features of patients before treatment, rating the overall inflammatory lesion severity of their rosacea via a 7-point static score, ranging from 0 (clear) to 6 (severe).
All patients completed an interview questionnaire that took 11 symptoms into account: diarrhea, upper and lower abdominal pain/discomfort, bloating, abdominal tenderness, nausea, vomiting, a burning or stinging pain when urinating, frequent urge to use the bathroom without being able to go, fever, and general feelings of being unwell. These symptoms were also scored from 0 (no symptoms) to 3 (severe). Based on these, a global symptomatic score (GSS), was calculated for each patient (with a maximum score of 33).
Prior to hydrogen and methane (HMBT) breath testing all subjects ate foods that were unlikely to generate hydrogen the day before, and then fasted overnight. The subjects did not smoke or undertake any exercise 1 hour before and during testing. Breath testing started after thorough mouth washing. Both a glucose and lactulose breath test were used, assessing clinically validated hydrogen and methane patterns in the breath for SIBO diagnosis. Patients positive for SIBO were randomized to receive 400 mg of rifaximin every 8 hours (n = 32) or a placebo (n = 20) for 10 days.
The patients then underwent a second breath test 1 month after stopping therapy to assess whether their SIBO had been successfully treated. Patients also completed a follow-up symptom questionnaire and dermatologic visit to assess the clinical features of their rosacea after treatment. Patients treated with a placebo were subsequently switched to rifaximin therapy.
Results
The HMBTs showed a significantly increased prevalence of SIBO in the patients with rosacea (52/113) compared with controls (3/60). SIBO was successfully treated, shown via a negative breath test in 28 of 32 patients (87.5%) that were treated with rifaximin, and this was associated with a significant decrease in the gastrointestinal symptoms experienced.
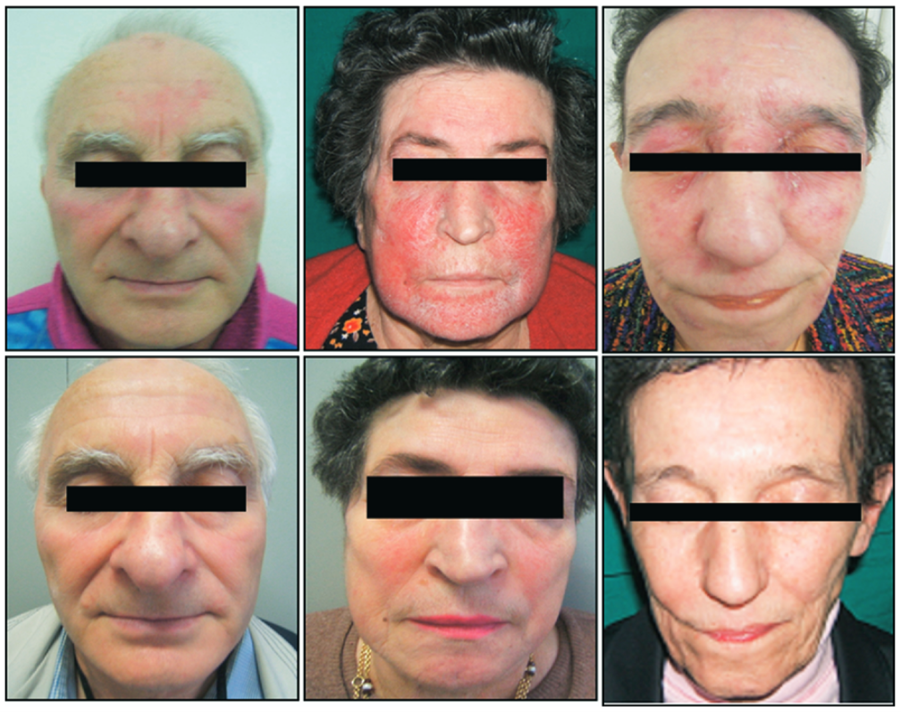
In 20 of the 28 patients who were successfully treated for SIBO, cutaneous lesions cleared (GA score, 0) in six patients (21.4%), papules and papulopustules were markedly reduced in number (GA score, 1), and the remaining two patients did not show any improvement. Figure 1 shows examples of successful reduction in rosacea symptoms in SIBO positive patients after rifaximin treatment.
Figure 1 – Selected rosacea patients with SIBO before and after treatment with rifaximin. All patients had complete resolution of cutaneous lesions after testing negative for SIBO via a hydrogen methane breath test (HMBT).
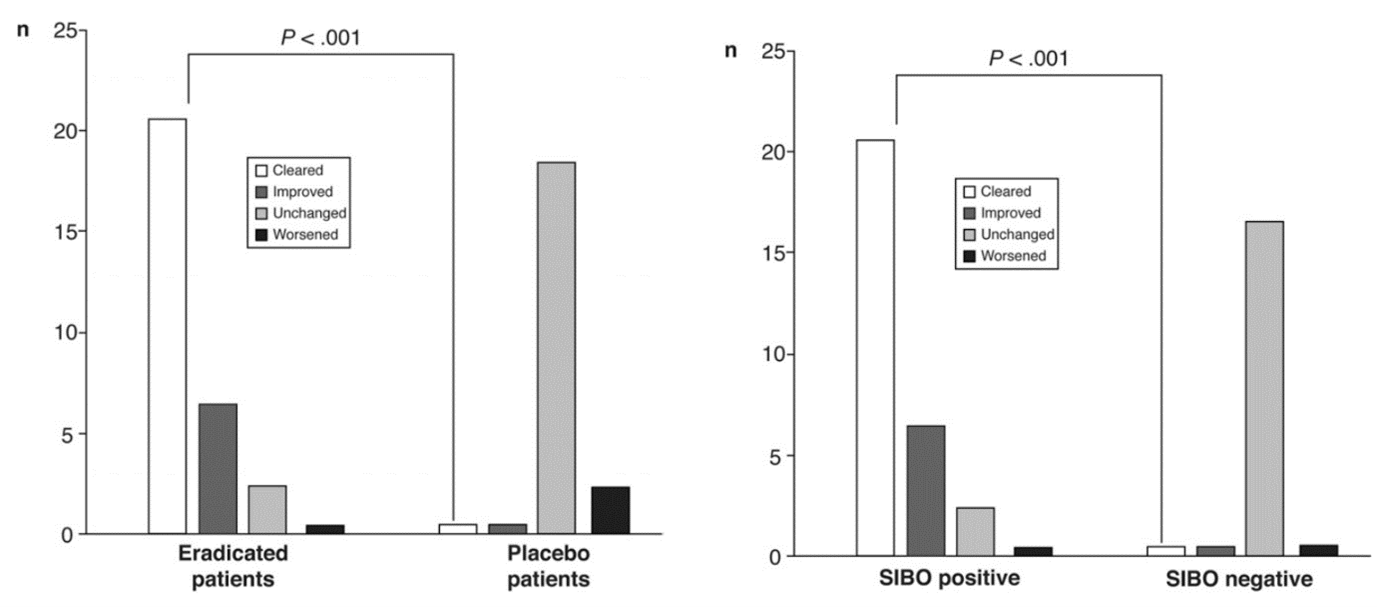
In the SIBO positive rosacea patients treated with a placebo, 18 of 20 patients (90%) showed unchanged rosacea symptoms in the follow-up appointment, and two patients (10%) had worse symptoms (Figure 2). Therefore, these 20 patients were subsequently treated with rifaximin, and 17 of them were successfully treated, rosacea lesions cleared in 15 patients with eradicated SIBO, and the two remaining cases showed a relevant improvement.
Figure 2 – Left: SIBO-positive patients treated with rifaximin (eradicated patients) or placebo, right: SIBO-positive and SIBO-negative patients treated with rifaximin.
The patients that were successfully treated for SIBO were then followed up for at least nine months, with all but two patients showing no recurrence of rosacea lesions. Interestingly, the two patients who were shown to be SIBO positive again, upon repeated rifaximin treatment, successfully cleared their rosacea recurrence.
Discussion
The results of this study suggest a mechanistic link between gut dysbiosis of the small intestine to the pathogenesis of rosacea. Rosacea patients had a significantly higher SIBO prevalence than controls, and treating this SIBO improved the lesions observed – and the majority of patients maintained this result for at least nine months. It is especially striking that all rosacea patients who experienced no change or worsening of their symptoms in the placebo group also showed a dramatic improvement in their lesions upon switching to the rifaximin-treated group.
The exact mechanism of this association is unclear, however, the authors of this study hypothesized that an increased intestinal permeability caused by SIBO could lead to the systemic circulation of increasing key immunomodulatory and pro-inflammatory compounds around the body that could activate an inflammatory response in the skin of certain people who are perhaps predisposed to rosacea.
Excessive bacteria in the small intestine indeed has been shown to have several pathological consequences such as direct mucosal injury, malabsorption, and decreased brush border enzyme activity, and other studies have shown that patients with SIBO often have increased endotoxin, and pro-inflammatory cytokines in their blood (15–17). SIBO therefore may trigger rosacea by the increase in these immunological factors in the body.
A strength of this study is that the poor absorbability of rifaximin means that it remains in the intestinal tract, and is barely absorbed for systemic effect. With the use of rifaximin (as opposed to antibiotics such as clarithromycin used in this study to treat H. pylori infection) there is very little interference with skin microbiota that could be having an impact on rosacea, or global anti-inflammatory effect that could be impacting the validity of the results. Indeed, patients without SIBO who undertook a course of rifaximin as part of the study did not show any improvement in their rosacea symptoms.
A limitation is that this study was not blinded, and therefore some bias could have impacted the results. However, the scoring of the two independent dermatologists was extremely similar, and the authors point out that the improvement was so striking that the scoring system used should not have been impacted by the lack of blinding.
Adding breath analysis to your microbiome research
This study demonstrates the power of breath analysis to provide key information about the activity of the gut microbiome through hydrogen gas. The levels of informative gases in the breath like hydrogen can be used to diagnose conditions such as SIBO that may have important roles in diseases of the skin like rosacea.
We can support clinical research by offering the analysis of the informative metabolites contained in breath, as well as portable hydrogen and methane breath analyzer devices that can support at-home collection of longitudinal data. The device can also provide a cheaper alternative to the analysis of breath using laboratory-based techniques like TD-GC-MS.
Beyond hydrogen and methane, there are hundreds of other informative compounds in the breath such as the short-chain fatty acids (SCFAs) that have been linked to other diseases that can be analyzed using our Breath Biopsy OMNI platform.
Our Breath Biopsy VOC Atlas is a catalog of confidently “on-breath” compounds (those that have been distinguished from contaminating background compounds) that can also be used as part of breath studies to provide a reference range of “normal” levels of microbially generated compounds, as well as evidence of mechanisms that produce the VOCs in the literature. If you have any questions or would like more information, please do not hesitate to contact us to discuss incorporating breath analysis in your research.
References
- Parada Venegas D, De la Fuente MK, Landskron G, González MJ, Quera R, Dijkstra G, et al. Short Chain Fatty Acids (SCFAs)-Mediated Gut Epithelial and Immune Regulation and Its Relevance for Inflammatory Bowel Diseases. Frontiers in Immunology. 2019;277.
- Frank DN, St. Amand AL, Feldman RA, Boedeker EC, Harpaz N, Pace NR. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc Natl Acad Sci U S A. 2007 Aug 21;104(34):13780–5.
- Zhu L, Baker SS, Gill C, Liu W, Alkhouri R, Baker RD, et al. Characterization of gut microbiomes in nonalcoholic steatohepatitis (NASH) patients: A connection between endogenous alcohol and NASH. Hepatology. 2013;57(2):601–9.
- Yuan J, Chen C, Cui J, Lu J, Yan C, Wei X, et al. Fatty Liver Disease Caused by High-Alcohol-Producing Klebsiella pneumoniae. Cell Metab. 2019 Oct 1;30(4):675-688.e7.
- Meijers BKI, Claes K, Bammens B, de Loor H, Viaene L, Verbeke K, et al. p-Cresol and Cardiovascular Risk in Mild-to-Moderate Kidney Disease. Clin J Am Soc Nephrol. 2010 Jul;5(7):1182–9.
- Saito Y, Sato T, Nomoto K, Tsuji H. Identification of phenol- and p-cresol-producing intestinal bacteria by using media supplemented with tyrosine and its metabolites. FEMS Microbiol Ecol. 2018 Jun 22;94(9):fiy125.
- Louis P, Hold GL, Flint HJ. The gut microbiota, bacterial metabolites and colorectal cancer. Nat Rev Microbiol. 2014 Oct;12(10):661–72.
- De Pessemier B, Grine L, Debaere M, Maes A, Paetzold B, Callewaert C. Gut–Skin Axis: Current Knowledge of the Interrelationship between Microbial Dysbiosis and Skin Conditions. Microorganisms. 2021 Feb 11;9(2):353.
- Rainer BM, Kang S, Chien AL. Rosacea: Epidemiology, pathogenesis, and treatment. Dermatoendocrinol. 2017 Oct 4;9(1):e1361574.
- Woo YR, Lim JH, Cho DH, Park HJ. Rosacea: Molecular Mechanisms and Management of a Chronic Cutaneous Inflammatory Condition. Int J Mol Sci. 2016 Sep 15;17(9):1562.
- Drabińska N, Flynn C, Ratcliffe N, Belluomo I, Myridakis A, Gould O, et al. A literature survey of all volatiles from healthy human breath and bodily fluids: the human volatilome. J Breath Res. 2021 Apr 21;15(3).
- Kendall SN. Remission of rosacea induced by reduction of gut transit time. Clinical and Experimental Dermatology. 2004 May 1;29(3):297–9.
- Sachdev AH, Pimentel M. Gastrointestinal bacterial overgrowth: pathogenesis and clinical significance. Ther Adv Chronic Dis. 2013 Sep;4(5):223–31.
- Parodi A, Paolino S, Greco A, Drago F, Mansi C, Rebora A, et al. Small Intestinal Bacterial Overgrowth in Rosacea: Clinical Effectiveness of Its Eradication. Clinical Gastroenterology and Hepatology. 2008 Jul 1;6(7):759–64.
- Sánchez-Pellicer P, Eguren-Michelena C, García-Gavín J, Llamas-Velasco M, Navarro-Moratalla L, Núñez-Delegido E, et al. Rosacea, microbiome and probiotics: the gut-skin axis. Front Microbiol. 2024 Jan 8;14:1323644.
- Dibaise JK, Young RJ, Vanderhoof JA. Enteric microbial flora, bacterial overgrowth, and short-bowel syndrome. Clin Gastroenterol Hepatol. 2006 Jan;4(1):11–20.
- Bures J, Cyrany J, Kohoutova D, Förstl M, Rejchrt S, Kvetina J, et al. Small intestinal bacterial overgrowth syndrome. World J Gastroenterol. 2010 Jun 28;16(24):2978–90.
Microbiome Content Pack: exclusive content on how breath analysis can be used for gut microbiome research